The following data have been reported for the osmotic pressure of -chymotrypsin in water at pH =
Question:
The following data have been reported for the osmotic pressure of α-chymotrypsin in water at pH = 8.0 in an 0.01 M potassium sulfate solution at 25°C.
Use these data to determine the molecular weight of α-chymotrypsin and the value of its osmotic second virial coefficient, B2, at these conditions.
Data:
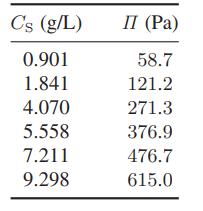
Transcribed Image Text:
C's (g/L) 0.901 1.841 4.070 5.558 7.211 9.298 П (Pa) 58.7 121.2 271.3 376.9 476.7 615.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To determine the molecular weight M of achymotrypsin and the value of its osmotic second virial coef...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
The following data have been reported for a sample of 10 major U.S. zoological parks: a. Determine the least-squares multiple regression equation. b. Interpret the y-intercept and partial regression...
-
The following data have been reported by Shen and Smith for benzene (Bz) adsorption on silica gel [16]: a) Do these data better fit a single site or a dual site Langmuir isotherm? Why? b) Assuming...
-
a. The following data have been reported for the vapor pressure of ethanol as a function of temperature. Use these data to calculate the heat of vaporization of ethanol at 17.33C. b. Ackermann and...
-
A mutual fund has 20 stocks in its portfolio. On a given day 9 stocks move up, 3 stay the same, and 8 move down. In how many ways could this happen?
-
Pinnacle Controls Corporation is in the business of leasing new sophisticated satellite systems. As a lessor of satellites, Pinnacle Controls purchased a new system on December 31, 2008. The system...
-
What do you understand by multi-valued attribute? How is it different from complex and composite attribute? Illustrate by giving suitable example.
-
What makes an organizations learning context conducive to successful training
-
1. What were the external and internal forces for change at HCL? 2. To what extent did Vineet Nayar follow the change models proposed by Lewin and Kotter? 3. Which of the target elements of change...
-
At the end of the previous year, a company's balance sheet reports cash of $30,000. For the current year, the company's statement of cash inflows of $90,000: Investing outflows of $110,000, and...
-
1. You will be able to use turbidity as a means of determining cell growth in future experiments. Using the plot of absorbance versus cell count derived in this exercise, you will be able to...
-
Derive the form of the Gibbs phase rule that applies to a multicomponent system in osmotic equilibrium in which all but two of the components can pass through the membrane separating the two...
-
Joe Udel lives on the second floor of a house that is adjacent to a well of pure water, but city water comes out of his indoor plumbing. He would rather have pure well water. So he has developed the...
-
Key figures for Apple and Google follow. Required 1. Compute times interest earned for the three years data shown for each company. 2. Comment on which company appears stronger in its ability to pay...
-
For each of the following events, indicate whether the freight terms are FOB destination or FOB shipping point. a. Sold merchandise and paid the freight costs. b. Purchased merchandise and paid the...
-
A converging mirror that has a radius of curvature of \(70.0 \mathrm{~mm}\) forms an image of an object that is \(20.0 \mathrm{~mm}\) tall and \(150 \mathrm{~mm}\) in front of the mirror. (a) What is...
-
Cramer Co. experienced the following events for the 2011 accounting period: 1. Acquired \(\$ 10,000\) cash from the issue of common stock. 2. Purchased \(\$ 18,000\) of inventory on account. 3....
-
Show that the Wald statistic in (4.15) does not depend on the specific equations used. Specifically, suppose that \(K\) and \(K^{\prime}\) are two equivalent systems of equations for a linear...
-
Mia Sales experienced the following events during 2011, its first year of operation: 1. Started the business when it acquired \(\$ 50,000\) cash from the issue of common stock. 2. Paid \(\$ 21,000\)...
-
Determine Zi Zo and Av for the network of Fig. 8.84. +20 V aSS 12 mA 3 91 = 45 k Z 10 MS2
-
Which task is performed by a book-keeper? A. Analysing the trading results B. Entering transactions in the ledger C. Preparing year-end financial statements D. Providing information for...
-
Which drying agent is more efficient, Drierite or phosphorus pentoxide?
-
The true volume of a 50-mL volumetric flask is 50.037 mL at 20C. What mass of water measured (a) in vacuum and (b) in air at 20C would be contained in the flask?
-
The efficiency of a gas chromatography column is measured by a parameter called plate height (H, mm) which is related to the gas flow rate (u, mL/min) by the van Deemter equation: H = A + B/u + Cu,...
-
Becker Office Service purchased a new computer system on January 1, Year 1, for $38,800. It is expected to have a five-year useful life and a $3,200 salvage value. Becker Office Service expects to...
-
Semcostadgrowng marutture of computer chi receded at the start of the production process Conversion cool added every ung the process. Some units of this prodare scored auto is not foto before medion...
-
Against - Lev and Gu (2016) All estimates are subject to errors Accounting estimates are sometimes manipulated by managers to make the numbers Argue that almost every income statement item and most...

Study smarter with the SolutionInn App


