The following equation of state describes the behavior of a certain fluid: where the constants are a
Question:
The following equation of state describes the behavior of a certain fluid:
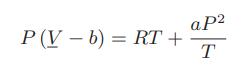
where the constants are a = 10−3 m3 K/(bar mol) = 102 (J K)/(bar2 mol) and b = 8 × 10−5 m3/mol. Also, for this fluid the mean ideal gas constant-pressure heat capacity, CP, over the temperature range of 0 to 300°C at 1 bar is 33.5 J/(mol K).
a. Estimate the mean value of CP over the temperature range at 12 bar.
b. Calculate the enthalpy change of the fluid for a change from P = 4 bar, T = 300 K to P = 12 bar and T = 400 K.
c. Calculate the entropy change of the fluid for the same change of conditions as in part (b).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





