The following set of reactions is thought to occur between nitrogen and oxygen at high temperatures a.
Question:
The following set of reactions is thought to occur between nitrogen and oxygen at high temperatures
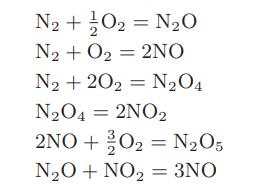
a. Find an independent set of reactions for the nitrogen-oxygen system.
b. How many degrees of freedom are there for this system?
c. If the starting oxygen-to-nitrogen ratio is fixed (as in air), how many degrees of freedom are there?
Transcribed Image Text:
N2+}O2 = N2O N₂ + O₂ = 2NO N₂ +202 N₂O4 N₂O4 = 2NO2 2NO+20₂ = N₂O5 N₂O + NO₂ = 3NO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The following set of reactions is thought to occur between nitrogen and oxygen at high temperatures ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
The following set of questions relates to using Poisson regression methods to analyze data from an in vitro study of human chromosome damage. In this study, using Poisson regression is appropriate...
-
Ann Carter, Chief Financial Officer of Consolidated Electric Company (Con El), must make a recommendation to Con Els board of directors regarding the firms dividend policy. Con El owns two...
-
Ivan Petrov was a member of the newly formed entrepreneur class in Bulgaria; partly out of desire, mostly out of necessity. The changes occurring as the country moved from a centrally planned to a...
-
On December 31, Alvare Company estimates its bad debts to be .5% of its annual credit sales of $875,000. They use the allowance method. Prepare the Journal entries for December 31 recording the Bad...
-
Jones bought a used car from the AHerts Car Rental System, which regularly sold its used equipment at the end of its fiscal year. First National Bank of Roxboro had previously obtained a perfected...
-
Kent County Council (KCC) governs the majority of the county of Kent, and comprises twelve District Councils and more than 300 town and Parish Councils. The Councils headquarters are based in...
-
What effect can the perceptual process have on individualsbehavior?
-
A stream of air at 500C and 835torr with a dew point of 30C flowing at a rate of 1515 L/s is to be cooled in a spray cooler. A fine mist of liquid water at 25C is sprayed into the hot air at a rate...
-
QUESTION 11 the US Dollar o prove to other countrs' currencies that means The USA produced exports are loss competitive for foreign buyers, thus increasing America's GDP The USA produced exports are...
-
[5] A marksman is firing a pistol at a 5 cm diameter, circular bullseye some distance away. The center of the target is considered (0, 0). This marksman pulls a little to the right (positive x) and a...
-
The molar integral heat of solution s H is defined as the change in enthalpy that results when 1 mole of solute (component 1) is isothermally mixed with N 2 moles of solvent (component 2) and is...
-
a. What is the maximum number of phases that can coexist for a mixture of two nonreacting components? b. How would the answer in part (a) change if the two components could react to form a third...
-
Describe your last two major and your last two minor purchases. What role did emotions or feelings play? How did they differ? What evaluative criteria and decision rules did you use for each? Why?
-
The following information pertains to the inventory of Parvin Company for Year 3: January 1 April 1 October 1 Beginning inventory 400 units @ $22 Purchased 2,600 units @ $27 Purchased 1,200 units @...
-
Using partial fraction decomposition to find the partial fractions of the function 26x2 +270x+404 f(x) = f(x) = 30x3 20x2-330x 280 Hint: The denominator of f(x) can be factored as (3x+7) (5x+5)(2x-8)...
-
Question 1: How do strategy and tactical action relate to each other in an organization? Question 2: How can you improve the implementation of strategy in this business organization?...
-
Question 1 Consider the function g (x) = x-6x+1. The discriminant is [Select] [Select] and therefore the graph has x-intercepts.
-
Han Wu Manufacturing uses a job order cost system and applies overhead to production on the basisof direct labour hours. On January 1, 2023, Job no, 50 was the only job in process. The costs incurred...
-
Determine Vo1, Vo2, and I for the network of Fig. 2.157. 0.47 k 20V S
-
SCHEDULE OF COST OF GOODS MANUFACTURED The following information is supplied for Sanchez Welding and Manufacturing Company. Prepare a schedule of cost of goods manufactured for the year ended...
-
The three forces (magnitudes 100 lb, 200 lb, and P) combine to produce a resultant R (Figure P4.9). The three forces act in known directions, but the numerical value P is unknown. (a) What should the...
-
Find a real physical example of a mechanical structure or machine that has a moment acting on it. (a) Make a clear, labeled drawing of the situation. (b) Estimate the dimensions, and the magnitudes...
-
Resulting from a light wind, the air pressure imbalance of 100 Pa acts across the 1.2-m by 2-m surface of the highway sign (Figure P4.11, see on page 172). (a) Calculate the magnitude of the force...
-
Kappa SA in 2021 had pre-tax profits of 100,000, equity 450,000 and return on equity 20%. How much did equity increase in 2021? Choose one: a. 100,000 b. Not at all c. None of the suggested answers...
-
I need help trying to find this information for Kroger grocery stores. I need the most recent year, list the amounts reported for sales, cost of goods sold, and total net income. Does the amount...
-
Your grandmother gives you 2400 dollars for your birthday, which you invest in a mutual fund on January 1. On June 1, your fund balance is 7200 dollars, and you then deposit 1100 dollars (which you...

Study smarter with the SolutionInn App


