The osmotic pressure for a small molecule in aqueous solution has been found to be given by
Question:
The osmotic pressure for a small molecule in aqueous solution has been found to be given by the following expression
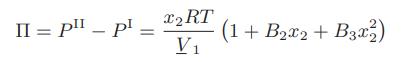
where 1 is the solvent and 2 is the small; molecule solute. Obtain expressions for the activity coefficients of the solvent and solute as a function of mole fraction.
Transcribed Image Text:
II = p _ pl - x2 RT V₁ (1 + B₂x2 + B3x2²2)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The osmotic pressure for a small molecule in aqueous solution can be expressed in terms of activity ...View the full answer

Answered By

AGRAHARAPU SAMPATH KUMAR
I applied to this website so as to do my tutoring job to put full efforts in it and improve my interested subject. I am interested in mathematics and this website has different questions to solve, so I applied to this as a tutor of mathematics.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
A 4 kg/s steady flow of ammonia runs through a device where it goes through a polytropic process. The inlet state is 150 kPa, -20oC and the exit state is 400 kPa, 80oC, where all kinetic and...
-
A 4 kg/s steady flow of ammonia runs through a device where it goes through a polytropic process. The inlet state is 150 kPa, -20oC and the exit state is 400 kPa, 80oC, where all kinetic and...
-
In manufacturing semiconductors, it is necessary to use water with an extremely low mineral content, and to separate water from contaminants, it is common practice to use a membrane process called...
-
The number x of bicycle helmets people are willing to buy per week from a retail chain at a price of $p is given by x = 1,000 - 60p + 25 20 ¤ p ¤ 100 (see the figure). (A) Find dx/dp....
-
In June 1988, British & Commonwealth PLC (B&C) acquired Atlantic Computers, the worlds third largest computer-leasing company. In April 1990, B&C placed Atlantic Computers into administrative...
-
What are adjusting entries? Why are they necessary for preparing final accounts?
-
Imagine you are working in a company where training is seen as a negativethat is, only incompetent employees need training. However, employees view going to diversity training as a punishment that...
-
Presented here are the components in Pedersen Companys income statement. Determine the missingamounts. Cost of Goods Sold Gross Profit $30,000 Operating Expenses Net ncomme $10,800 $29,500 Sales...
-
Question 12 5 pts The management of Solar Corporation is considering the following three investment projects (Ignore income taxes): Project L Project M Project N Investment require $ 37,000 $ 55,000...
-
1. What was (a) the return on assets in 2021 and (b) the average return on assets for the most recent five years (rounded to the nearest one-half percent) for Tru, Inc.? 2. What was (a) the cash...
-
The more environmentally friendly refrigerants are made of hydrogen-containing compounds that have a short environmental lifetime. However, these compounds are also combustible. Therefore, one...
-
A new membrane premeable only to water is to be used for purifying water containing a pesticide. Your employer requests that you setup a demonstration consisting of an insulated rigid tank at 2C...
-
Dad gives Son a 20% capital and profits interest in the Family Partnership. Dad holds a 70% interest, and Fred, an unrelated individual, holds a 10% interest. Dad and Fred work in the partnership,...
-
Consider Figure 9-9, panel (b). Based on the data there, which regions support the convergence hypothesis? Which do not? Explain. FIGURE 9-9 Do Economies Converge? (b) But Not for the World as a...
-
Draw a diagram with AD, SRAS and LRAS. Be careful to label the axes correctly.
-
Keeping the settings of the Dang-Gorton-Holmstrm-Ordoez model mostly unchanged, except that - The bank is the only financial firm in the economy, that provides a mixture of deposit contract and bond...
-
Implement the below function for the queue: a. IsEmpty: This is left as an exercise for the user. Take a variable, which will take care of the size of a queue if the value of that variable is zero,...
-
Check whether a given Binary Tree is Full/ Strictly binary tree or not. The full binary tree is a binary tree in which each node has zero or two children. 3 5 00 8
-
Design the fixed-bias network of Fig. 8.88 to have a gain of 8. +V DD (+22 V ) IDSS8 mA 10
-
The water in tank A is at 270 F with quality of 10% and mass 1 lbm. It is connected to a piston/cylinder holding constant pressure of 40 psia initially with 1 lbm water at 700 F. The valve is opened,...
-
Write the names and abbreviations for each of the prefixes from 10 -24 to 10 24 . Which abbreviations are capitalized?
-
What is the formal concentration (expressed as mol/L = M) of NaCl when 32.0 g are dissolved in water and diluted to 0.500 L?
-
How many grams of boric acid, B(OH) 3 (FM 61.83), should be used to make 2.00 L of 0.050 0 M solution? What kind of flask is used to prepare this solution?
-
A farmer is concerned that the price of wheat will drop by the time he is ready to sell his crop. He, therefore, enters into a futures contract on 5,000 bushels of wheat for 250 cents per bushel. The...
-
On December 1, ABC Company received $3,000 cash from a customer for 3 months of business services beginning December 1st. Prepare the journal entry to record the receipt of the 3,000 and the...
-
When Kevin started working 23 years ago, his salary was $59,349. His current salary is $159,408. When Kevin started working, the price level was 134, while the current price level is 157. What was...

Study smarter with the SolutionInn App


