We want to make a simplified estimate of the maximum amount of work that can be obtained
Question:
We want to make a simplified estimate of the maximum amount of work that can be obtained from gasoline, which we will assume to be adequately represented by n-octane (C8H18). The processes that occur in the cylinder of an automobile engine are that first the gasoline reacts to form a high-temperature, high-pressure combustion gas consisting of carbon dioxide, water, and the nitrogen initially present in the air (as well as other by-products that we will neglect), and then work is extracted from this combustion gas as its pressure and temperature are reduced.
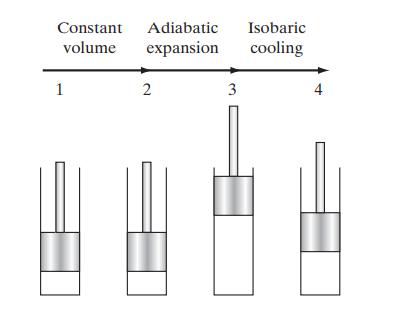
a. Assuming that n-octane (vaporized in the fuel injector) and a stoichiometric amount of air (21 vol. % oxygen, 79 vol. % nitrogen) initially at 1 bar and 25°C react to completion at constant volume, calculate the final temperature and pressure of the combustion gas, assuming further that there is no heat loss to the pistons and cylinders of the automobile engine.
b. Calculate the final temperature of the combustion gas and the work that can be obtained from the gas if it is adiabatically expanded from the pressure found in part (a) to 1 bar.
c. Calculate the maximum amount of additional work that can be obtained from the combustion gas as its temperature is isobarically lowered from the temperature found in part (b) to an exhaust temperature of 150°C.
Data: Consider the gas to be ideal, in which case the partial molar enthalpies of the components are equal to their pure component molar enthalpies at the same temperature and pressure.
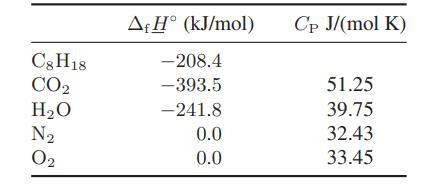
Note that for the calculations here the molar heat capacity of the mixture is just the mole fraction– weighted sum of the heat capacities of the individual components,
Below is a diagram of the three steps in the process.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





