Wilson has proposed that the excess Gibbs energy of a multicomponent system is given by Note
Question:
Wilson has proposed that the excess Gibbs energy of a multicomponent system is given by
![where Gex == - RT ;; ; 1n ;4;; i=l = (Xij- Aii) ] exp RT](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/1/3/1/0246555f0d0528091700131023749.jpg)
Note that this equation contains only the interaction parameters Λij for binary mixtures. Also, the parameters (λij −λii) appear to be insensitive to temperature. Holmes and van Winkle have tested this equation and found it to be accurate for the prediction of binary and ternary vapor–liquid equilibria. They also report values of the parameters VLi and (λij − λii) for many binary mixtures. Use the Wilson equation to
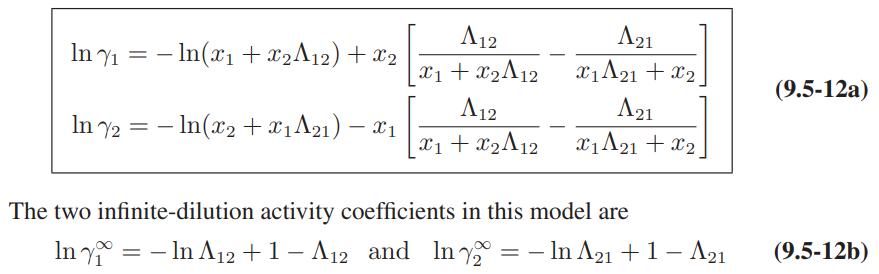
a. Derive Eqs. 9.5-12 and 9.5-13 for the activity coefficients of a species in a binary mixture.
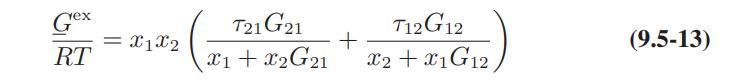
b. Obtain the following expression for the activity coefficient of species 1 in a multicomponent mixture
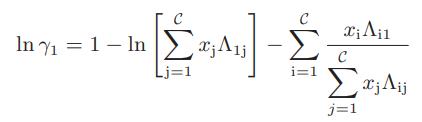
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





