Question: A flash drum at 700.0 kPa is separating binary mixtures of ethane and n-butane. a. The following equilibrium results were generated using correct units in
A flash drum at 700.0 kPa is separating binary mixtures of ethane and n-butane.
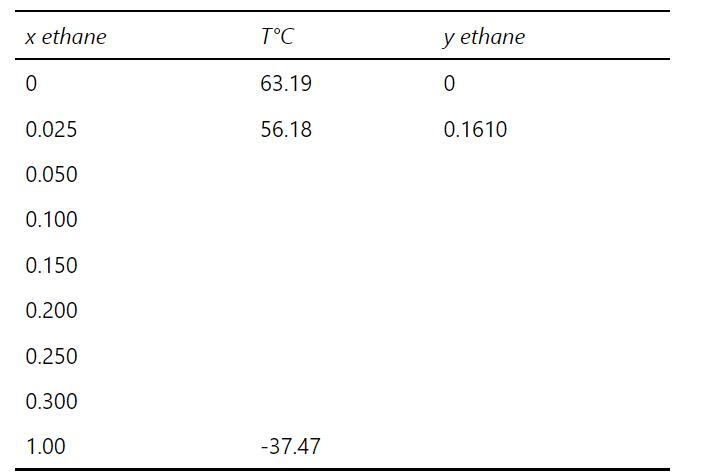
a. The following equilibrium results were generated using correct units in Eq. (2-28) and then converting temperature to C. Complete the table and use the results to answer the questions that follow.
Eq (2-28)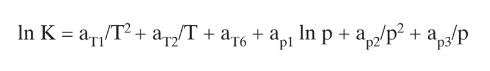
 Use these equilibrium results to answer the following:
Use these equilibrium results to answer the following:
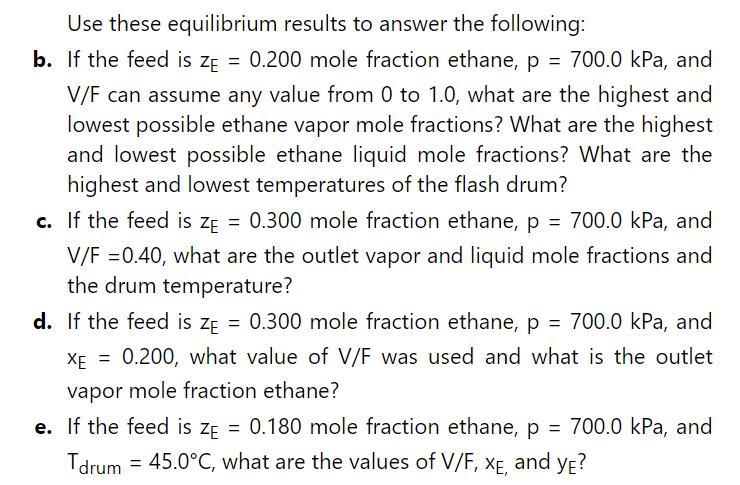
b. If the feed is ZE = 0.200 mole fraction ethane, p = 700.0 kPa, and V/F can assume any value from 0 to 1.0, what are the highest and lowest possible ethane vapor mole fractions? What are the highest and lowest possible ethane liquid mole fractions? What are the highest and lowest temperatures of the flash drum?
c. If the feed is ZE = 0.300 mole fraction ethane, p = 700.0 kPa, and V/F = 0.40, what are the outlet vapor and liquid mole fractions and the drum temperature?
d. If the feed is ZE = 0.300 mole fraction ethane, p = 700.0 kPa, and XE =0.200, what value of V/F was used and what is the outlet vapor mole fraction ethane?
e. If the feed is ZE = 0.180 mole fraction ethane, p = 700.0 kPa, and Tdrum 45.0C, what are the values of V/F, XE, and YE?
In K = a/T+ a2/T + 6 a2/T + a + a In p+ a2/p + a/p pl p3
Step by Step Solution
3.40 Rating (144 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


