Question: An example of a problem where there is severe counter-diffusion of the products is the deposition of (mathrm{SiO}_{2}) from tetraethoxysilane (TEOS) on a solid substrate.
An example of a problem where there is severe counter-diffusion of the products is the deposition of \(\mathrm{SiO}_{2}\) from tetraethoxysilane (TEOS) on a solid substrate. The reaction is represented as
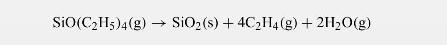
Note that 6 moles have to counter-diffuse and hence this retards mass transfer unless the mole fraction of TEOS is small. Consider deposition from a gas at a temperature of \(400 \mathrm{~K}\) and pressure \(0.1 \mathrm{~atm}\) and a mole fraction of TEOS of 0.2 . The diffusivity is \(0.1 \mathrm{~cm}^{2} / \mathrm{s}\). Calculate the rate of reaction and the film growth rate assuming that the surface reaction is very rapid.
SiO(C2H5)4(g) SiO2 (s) + 4C2H4(g) + 2H2O(g)
Step by Step Solution
3.40 Rating (147 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


