Find the heat of reaction H R for each of the following reactions using both heat of
Question:
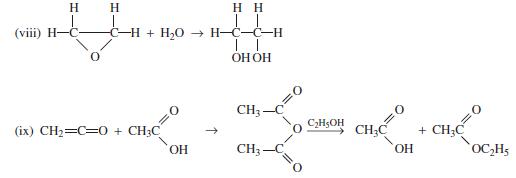
Find the heat of reaction ΔHR for each of the following reactions using both heat of formation and heat of combustion data, as available.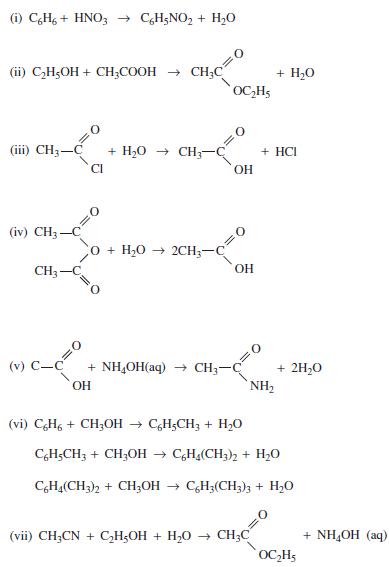
Transcribed Image Text:
(1) C6H6+ HNO3 → C6H5NO₂ + H₂O (ii) C₂H5OH + CH₂COOH → CH₂C (iii) CH3-C + H,O → CH CI (iv) CH3-C CH3- (v) C-C O + H,O → 2CH + NH₂OH(aq) → CH₂- OH OC₂H₂ OH OH (vi) C&H6 + CH3OH → C6H₂CH3 + H₂O NH₂ + H₂O + HCI (vii) CH3CN + C₂H5OH + H₂O → CH3C + 2H₂O C6H5CH3 + CH₂OH→ C6H4(CH3)2 + H₂O C6H4(CH3)2 + CH3OH → C6H3(CH3)3 + H₂O OC₂H5 + NH,OH (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To calculate the heat of reaction HR for each chemical reaction you can use either the heats of form...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
a. Derive an equation for the heat of reaction at any temperature if you are given the heat of reaction H R R(T o ) at a standard temperature T o . You may assume an ideal solution. b. Find the...
-
Ethyl alcohol (ethanol) can be produced by the fermentation of sugars derived from agricultural products such as sugarcane and corn. Some countries without large petroleum and natural gas...
-
Ethyl alcohol (ethanol) can be produced by the fermentation of sugars derived from trains and other agricultural products. Some countries without large petroleum and natural as reservessuch as...
-
Translate the seven steps to Java code.
-
Explain why inherent risk is set for segments rather than for the overall audit. What is the effect on the amount of evidence the auditor must accumulate when inherent risk is increased from medium...
-
What carbonyl compounds give the following alcohols on reduction with LiAlH4? Show allpossibilities. (a) CH- (b) CHCH3 (d) (CH)2HCH20 (c)
-
Which is the least appropriate? The main headings may appear at the top of the internal control evaluation schedule as: a. Systems objectives b. Control objectives c. Risks to the achievement of...
-
A brass rod 100 mm long and 5 mm in diameter extends horizontally from a casting at 200e. The rod is in an air environment with T = 20C and h = 30 W/m2 K. What is the temperature of the rod 25, 50,...
-
Brian Knight Corporation operates four bowling alleys. The business just received the October 31, 2018, bank statement from City National Bank, and the statement shows an ending balance of $900....
-
a. 5 kg/hr of sodium hydroxide and 10 kg/hr of water are to be mixed in an insulated tank. Both feed streams are to be put into the mixer at the same temperature. What is a safe inlet temperature at...
-
A continuous mixing operation is carried out by adding 0.3 kg/hr of calcium chloride crystals to a pure water stream flowing at 1 kg/hour. a. If both streams are at 25C when they enter the mixer,...
-
Consider a municipal water treatment plant for a small community (Fig. P1.1). Waste water, 32,000 m 3 /day, flows through the treatment plant with a mean residence time of 8 hr, air is bubbled...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
I need help for an assignment of a review on research on Virtual Education on study motivation and academic performance in university students. I am attaching a research article from a magazine to...
-
Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this small...
-
Required information Use the following information for the Exercises below. (Algo) [The following information applies to the questions displayed below.] Ramirez Company installs a computerized...
-
Reproduced below from Farthington Supply s accounting records is the accounts receivable subledger along with selected general ledger accounts. General Ledger Accounts Receivable Dec. 3 1 / 2 2...
-
Craft Pro Machining produces machine tools for the construction industry. The following details about overhead costs were taken from its company records, costing Additional information on the drivers...
-
A local politician is concerned that a program for the homeless in her city is discriminating against blacks and other minorities. The following data were taken from a random sample of black and...
-
If the sucrose concentration is 20% what will be the flow rate at the higher pressure in the previous problem.
-
A solution of sucrose in water is concentrated by RO. It is found that, with a differential applied pressure of 6000 kPa, the rate of movement of the water molecules through the membrane is 25 kg m2...
-
Derive the equation for Langmuir adsorption isotherm.
-
oFly Corporation sells three different models of a mosquito zapper. Model A12 sells for $50 and has variable costs of $35. Model B22 sells for $100 and has variable costs of $70. Model C124 sells for...
-
In the Illustrative Case in this chapter, payroll transactions for Brookins Company were analyzed, journalized, and posted for the third quarter of the fiscal year. In this problem, you are to record...
-
The Great Gumball Corporation is a gumball producer. The corporation has accumulated earnings of $425,000 and it can establish a reasonable need for $360,000. Calculate the amount of the accumulated...

Study smarter with the SolutionInn App


