What carbonyl compounds give the following alcohols on reduction with LiAlH4? Show allpossibilities. (a) CH- (b)
Question:
What carbonyl compounds give the following alcohols on reduction with LiAlH4? Show allpossibilities.
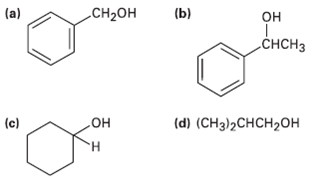
Transcribed Image Text:
(a) CH-он (b) он „CHCH3 (d) (CHз)2СHCH20Н (c) но
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
i OH obotowor a CCH3 1 LIAIH4 2H30 d Benzyl alcohol may b...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show what alcohols and carbonyl compounds give the following derivatives. (a) (b) (c) (d) (e) (f) CH CH,O OCH,CH CH O-CH CH3 CH3 CH-C H O-CH CH a,0 OX
-
Show what amines and carbonyl compounds combine to give the following derivatives. (a) (b) (c) (d) (e) (f) Ph-CH=N-NH-C-NH, NOH N NHPh HNN : O
-
What carbonyl compounds are required to prepare a compound with molecular formula C10H10Owhose spectrum is shown? (ppm)
-
On 1 June 1998, Alice bought a house in Derby for 45,000. - She occupied the house as her PPR until 1 May 2000 when she left to work in Exeter, living in rented accommodation. - She returned to the...
-
Illustrate a policy, an objective, and a functional tactic in your personal career strategy.
-
1. Describe the flaws in Targets security system that enabled the breach. 2. Was Targets response to the breach appropriate? Why or why not? 3. What should you do as a consumer to protect yourself...
-
P6-6 Workpaper (downstream and upstream sales) Pam Corporation acquired all the outstanding stock of Sun Corporation on April 1, 2016, for $15,000,000, when Suns stockholders equity consisted of...
-
On January 1, 2017, Four Brothers Manufacturing borrowed $10 million from Guiffrie Bank by signing a three-year, 8.0% fixed-rate note. The note calls for interest to be paid annually on December 31....
-
4. Students will comment on the bailouts of banks and sovereign risks focusing on the subsequent Eurozone crisis. What precipitated it and what actions had been taken to sustain the Euro?
-
Print-for-All is a family-owned print shop that has grown from a three-press two-color operation to a full-service facility capable of performing a range of jobs from simple copying to four-color...
-
What reagent would you use to accomplish each of the following reactions? , 2. (a) CHCH-CH2co CHH2CH2cH , 2. (b) CHH2H2H2 CHH2H2cH (c)
-
Show the products obtained from addition of methyl magnesium bromide to the following compounds: (a) Cyclopentanone (b) Benzophenone (diphenyl ketone) (c) 3-Hexanone
-
In the common-base circuit shown in Figure P7.70, the transistor parameters are: \(\beta=100, V_{B E}(\) on \()=0.7 \mathrm{~V}, V_{A}=\infty, C_{\pi}=10 \mathrm{pF}\), and \(C_{\mu}=1 \mathrm{pF}\)....
-
A farmer has an acre of specialty vegetables and is preparing for the summer harvest. Historically, this acre has yielded an average of 2,100 lbs of product with a standard deviation of 950 lbs. A...
-
Solve 3x 82+22 = (4).
-
(c) Compute EVPI and EVSI (in thousands of dollars). (Round your answers to one decimal place.) EVPI $ 3.6 EVSI $ 3.6 Xthousand x thousand Discuss whether the firm should consider a consulting expert...
-
Question 9 (1 point) If the common law requires employees of a bar establishment to monitor a potentially intoxicated patron and to possibly make an effort to intervene if there is an indication the...
-
B. A velocity potential is given by the equation: Q = x-y 3. (10 pts) Short answer, What special characteristics of the velocity potential make it very useful in identifying a type of flow and...
-
Identify the purposes for the various types of budgets used in the hospitality industry.
-
Floyd Distributors, Inc., provides a variety of auto parts to small local garages. Floyd purchases parts from manufacturers according to the EOQ model and then ships the parts from a regional...
-
Construct a concept map using the ideas of packing of spheres and the structure of metal and ionic crystals.
-
Starting with appropriate unlabeled organic compounds, show syntheses of each of the following: (a) C6H5-C¡C-T (b) (c) CH3CH2CH2OD CH3
-
(a) Arrange the following compounds in order of decreasing acidity and explain your answer: CH3CH2NH2, CH3CH2OH, and CH3CH2CH3. (b) Arrange the conjugate bases of the acids given in part (a) in order...
-
Arrange the following compounds in order of decreasing acidity: (a) CH3CH==CH2, CH3CH2CH3, CH3CCH (b) CH3CH2CH2OH, CH3CH2CO2H, CH3CHClCO2H (c)
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...

Study smarter with the SolutionInn App


