What reagent would you use to accomplish each of the following reactions? , 2. (a) CHCH-CH2co
Question:
What reagent would you use to accomplish each of the following reactions?
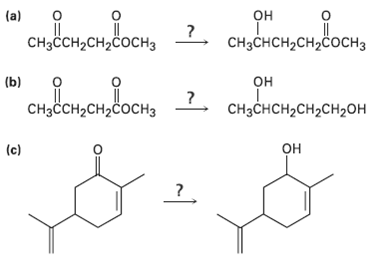
Transcribed Image Text:
обенодо, 2. ореородвонть (a) CHзснCH-CH2coснз CHзссH2CH2cосHз сононвоси, 2. он (b) CHзснсH2сH2сH2он CHзҫсH2сH2cосHз он (c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (16 reviews)
1 NaBH4 2 H30 OH CH3CHCHCHCOCH 3 NaBH4 reduces aldehydes and k...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What reagents would you use to accomplish a substitution with retention of configuration; for example? OH SH (R)-2-Butanol (R)-2-Butanethiol
-
Identify the reagent you would use to accomplish each of the following transformations: (a) Cyclobutanol bromocyclobutane (b) tert-Butanol tert-butyl chloride (c) Ethyl chloride ethanol
-
Each of the following reactions has been reported in the chemical literature and proceeds cleanly in good yield. Identify the principal organic product in each case. (a) (b) (c) (d) (e) (f) (g) (h)...
-
Alistair bought a house on 1 April 2000 for 125,000 and occupied the entire house as his principal private residence until 1 November 2008. As from that date, he rented out two rooms (comprising...
-
Why are short-term objectives needed when long-term objectives are already available?
-
Hyundais Global Command and Control Center (GCCC) have cameras strategically placed across its centers to monitor assembly lines. This helps identify problems and respond quickly. What drives Hyundai...
-
4. Pop uses the equity method to accounting for its investment in Son. REQuIRED: Prepare a consolidation workpaper for Pop Corporation and Subsidiary for the year ended December 31, 2016.
-
DryIce Inc., is a manufacturer of air conditioners that has seen its demand grow significantly. The company anticipates nationwide demand for the next year to be 180,000 units in the South, 120,000...
-
Dorsey Company manufactures three products from a common input in a joint processing operation. Joint processing costs up to the split - off point total $ 3 2 0 , 0 0 0 per quarter. For financial...
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
Predict the products of the following reactions: CH (a) 1. CHCH2- 2. NaOH, H2O2 "CH (b) 1. HglOAc)2. 0 2. NABH4 (c) CCH2CH2CH2 CH2CH2CH2CH3 C=C 1. Os04 2. NaHSO3, H20 -
-
What carbonyl compounds give the following alcohols on reduction with LiAlH4? Show allpossibilities. (a) CH- (b) CHCH3 (d) (CH)2HCH20 (c)
-
Ursula is a wholesaler trading in stationery supplies. She sells to offices and shops around the country and at any one time has up to 350 debtors due to pay her. She allows 30 days credit but finds...
-
! Required information [The following information applies to the questions displayed below.] Littleton Books has the following transactions during May. May 2 Purchases books on account from Readers...
-
4) Consider the table to the right that shows the number of free samples () and number of protein shakes sold (y). a)Complete the table [3 marks] b) Find the equation of the line of best fit X x y 8...
-
Determine the mean number of credit cards based on the raw data. (b) Determine the standard deviation number of credit cards based on the raw data. (c) Determine a probability distribution for the...
-
B Harry is a county Department of Social Services worker whose clients consist primarily of poor, female-headed families receiving public assistance. During one of his meetings with Dora, a single...
-
1 A, Weakly coupled carts (20 points) m m2 Figure 1: A system of two masses and three springs. A symmetric two degree of freedom system consists of two identical rigid masses m = m = m pictured in...
-
Trisha Hanes purchased Casa del Sol, an 85-room full-service hotel on the Pacific coast in Nicaragua, in 2006. She felt then she was ahead of her time when she made the purchase and now she is...
-
What types of inventory issues Starbucks might reflect upon at the end of each year? The mission of Starbucks is to inspire and nurture the human spiritone person, one cup, and one neighborhood at a...
-
Construct a concept map showing the ideas contained in a phase diagram.
-
What reaction will take place if ethyl alcohol is added to a solution of HCC:- Na+ in liquid ammonia?
-
Acid HA has pKa = 20; acid HB has pKa = 10. (a) Which is the stronger acid? (b) Will an acid-base reaction with an equilibrium lying to the right take place if Na+A- s added to HB? Explain your...
-
Which of the following are potential Lewis acids and which are potential Lewis bases? (a) (b) (c) (C6H5)3P: (d) (e) (CH3)3B (f) H: - CH.CH-N-CH CH3 CH3 H3C-C CH Br:
-
Questien It Calraluta bae neark yoe cen atforal to berren
-
In calculating the net present value of a proposed project, the cash flows of the project should include a.) amortization of goodwill b.) interest expenses paid to bondholders c.) extra working...
-
If Yolanda's insurance company cancels her fire insurance policy after 204 days, how much of the $682.00 annual premium will she receive as a refund (in $)? (Round you answer to the nearest cent.) $

Study smarter with the SolutionInn App


