Here are data from a (mathrm{pH}) titration of potassium hydrogen phthalate with (mathrm{NaOH}) using an automated titrator.
Question:
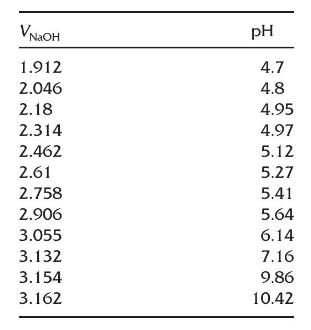
Here are data from a \(\mathrm{pH}\) titration of potassium hydrogen phthalate with \(\mathrm{NaOH}\) using an automated titrator. Only data for part of the titration has been provided. Prepare a Gran plot and find the equivalence point volume.
Transcribed Image Text:
NGOH 1.912 pH 4.7 4.8 2.046 2.18 4.95 2.314 4.97 2.462 5.12 2.61 5.27 2.758 5.41 2.906 5.64 3.055 6.14 3.132 7.16 3.154 9.86 3.162 10.42
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
VNaoH pH VNaoH10PH 0000045 1912 47 38149E05 2046 218 48 32427E05 495 2446E05 2314 2462 49...View the full answer

Answered By

Mario Alvarez
I teach Statistics and Probability for students of my university ( Univerisity Centroamerican Jose Simeon Canas) in my free time and when students ask for me, I prepare and teach students that are in courses of Statistics and Probability. Also I teach students of the University Francisco Gavidia and Universidad of El Salvador that need help in some topics about Statistics, Probability, Math, Calculus. I love teaching Statistics and Probability! Why me?
** I have experience in Statistics and Probability topics for middle school, high school and university.
** I always want to share my knowledge with my students and have a great relationship with them.
** I have experience working with students online.
** I am very patient with my students and highly committed with them
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Electroanalytical Chemistry Principles Best Practices And Case Studies
ISBN: 9781119538592,9781119538585
1st Edition
Authors: Gary A. Mabbott
Question Posted:
Students also viewed these Engineering questions
-
A solution of NaOH was standardized by titration of a known quantity of the primary standard, potassium hydrogen phthalate The NaOH was then used to find the concentration of an unknown solution of H...
-
A solution of NaOH was standardized by gravimetric titration of a known quantity of the primary standard, potassium hydrogen phthalate: Potassium hydrogen phthalate C 8 H 5 O 4 K, FM 204.22 The NaOH...
-
Potassium hydrogen phthalate is a primary standard used to measure the concentration of NaOH solutions. Find the true mass of potassium hydrogen phthalate (density = 1.636 g/mL) if the mass weighed...
-
Assume that the average talk time on an Apple iPhone is 20 hours and that this time follows the exponential probability distribution. What is the probability that a randomly selected iPhone will...
-
Tyler and Stephanie work for a direct marketing firm. They make calls to customers for a local carpet cleaning service. In a typical hour, Tyler completes 50 calls and gets two sales Stephanie...
-
Stronger, leaner organisations are better able to compete in a global economy; however, this may mean workers face job losses. Is this system fair?
-
Recognize why everyone needs to learn how to lead today. (p. 56)
-
Bonka Toys is considering a robot that will cost $20,000 to buy. After 7 years its salvage value will be $2000. An overhaul costing $5000 will be needed in year 4. O&M costs will be $2500 per year....
-
During 2020, which was the first year of operations, Sheridan Company had merchandise purchases of $992000 before cash discounts. All purchases were made on terms of 2/10, 1/30. Three-fourths of the...
-
Why is the filling port for the outer reference chamber of a combination glass electrode kept open during measurements?
-
Imagine that you were asked to set up a flow injection system in order to measure \(\mathrm{K}^{+}\)ion levels in blood samples in a hospital lab. List at least five different challenges for...
-
a. What is the objective of internal auditing? b. The scope of internal auditing is limited to financial statement audits. Do you agree? Explain.
-
Coaching for Performance Develop a strategy for how you will approach the coaching session with the employee, including what you plan to discuss and any questions you may have when you debrief....
-
For the following exercises, find the derivatives of the given functions: 1. y=x-secx+1 2. y = 3 cscx+ 5 3. f(x) = x cotx 4. f(x) = secx I 5. y=
-
1. why does Amazon use ERP system? How does ERP system work for Amazon? what are the benefit and drawbacks of using ERP for Amazon? 2. what are 5 industry best practices across Finance,...
-
A rigid vessel contains afuel gasconsisting of a methane (CH4) and ethane (C2H6) mixture. The pressure in the vessel is found to be 0.30 bar.Air is added to the vessel until the total pressure...
-
Do you agree with this discussion post? My article discusses decision-making tools in Project Management (PM). "In research and development (R&D), project management (PM) decision-making tools are...
-
Suppose that the market for e-readers is an oligopoly controlled by Amazon.com, Barnes and Noble, Sony, and Apple. Barnes and Noble is considering increasing its output. How would this affect the...
-
A new car sold for $31,000. If the vehicle loses 15% of its value each year, how much will it be worth after 10 years?
-
What will be change in the heat transfer coefficient in problem 2 after the modification? Data from in problem 2 In a rising film evaporator, if the heat transfer coefficient is proportional V0.6,...
-
In a rising film evaporator, if the heat transfer coefficient is proportional V0.6, where V is the rising vapor velocity. If the throughput through the reactor has to be increased by 25%, how many...
-
How long it will take to reduce the pressure of a 100 L tank from 1 Kg/cm2 (a) to 0.2 Kg/cm2 (a), if the pump capacity is 500 L/min. assume 10% leakage.
-
How does the life time analysis differ from the basic customer profitability approach(500 words)
-
The security analysis research reports, published by sell side analysts working for security firms and FINRA, purport to sell an analyst's investment ideas to an investor in exchange for...
-
During the month of September,the Cider Pressing Company is trying to determine how much cider they are going to sell in October and November. One gallon of cider typically sells for $7 per gallon....

Study smarter with the SolutionInn App


