Since sodium sulfate has reverse solubility, an evaporative crystallizer operating at (100^{circ} mathrm{C}) is used to concentrate
Question:
Since sodium sulfate has reverse solubility, an evaporative crystallizer operating at \(100^{\circ} \mathrm{C}\) is used to concentrate and crystallize \(1500 \mathrm{~kg} / \mathrm{h}\) of an aqueous feed at \(100^{\circ} \mathrm{C}\) that contains \(28.57 \mathrm{wt} \%\) sodium sulfate. Data are in Table 17-1.
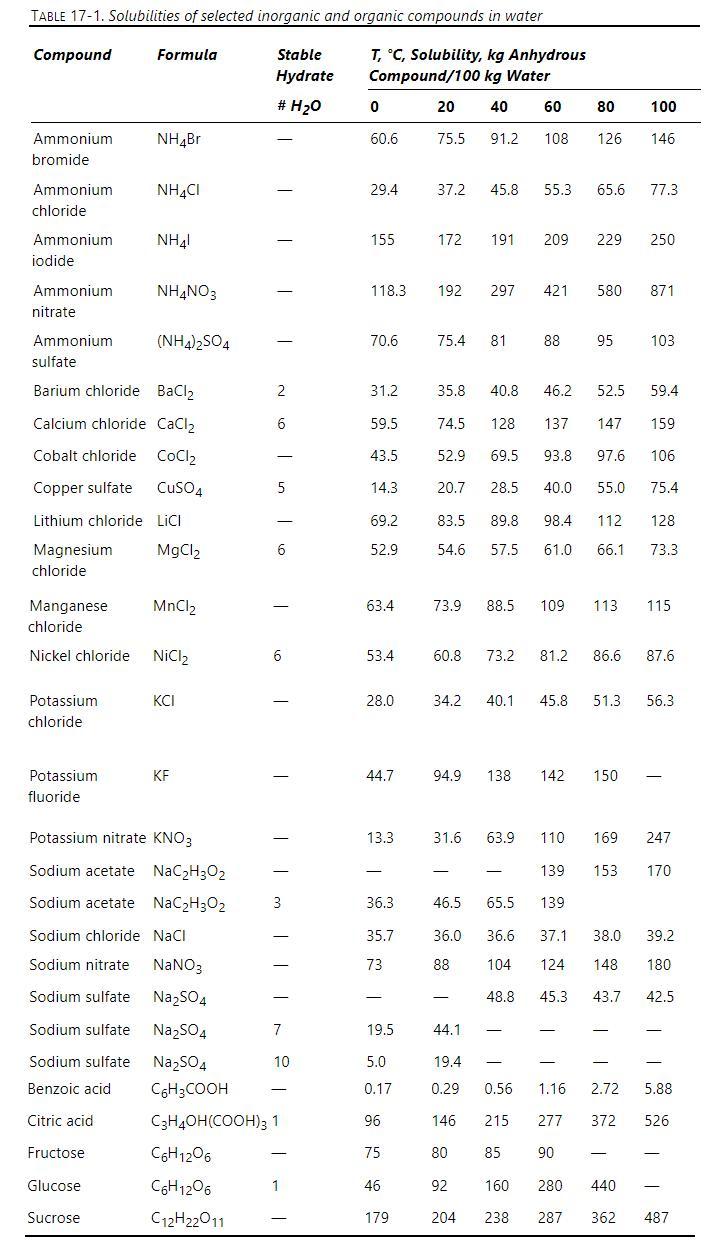
a. How much vapor needs to be removed \((\mathrm{kg} / \mathrm{h})\) to saturate the feed?b. After step
a, we remove an additional \(550 \mathrm{~kg} / \mathrm{h}\) of vapor. How many \(\mathrm{kg} / \mathrm{h}\) of anhydrous crystals are obtained?
c. What is \(\mathrm{F}_{\mathrm{W}, \text { out }}\) ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:






