Question: Some azeotropic systems can be separated by operating two coupled distillation columns at different pressures. The azeotrope concentration shifts enough that pure products can be
Some azeotropic systems can be separated by operating two coupled distillation columns at different pressures. The azeotrope concentration shifts enough that pure products can be recovered from the bottom of each column. This arrangement, shown in Figure 8-8, is used for the separation of water and MEK (methyl ethyl ketone). The saturated liquid feed is 100 \(\mathrm{kmol} / \mathrm{h}\) of \(60 \mathrm{~mol} \%\) water and \(40 \mathrm{~mol} \% \mathrm{MEK}\) at \(1.0 \mathrm{~atm}\). Column 1 operates at \(1.0 \mathrm{~atm}\). Column 2 is at 100 psia. Both columns have total condensers and partial reboilers. MEK is most volatile in column 1 the bottoms from column 1 is 0.0007 mole fraction MEK, and the distillate from column 1 (which is feed to column 2) is 0.6893 mole fraction MEK. Water is most volatile in column 2, the bottoms from column 2 is 0.0003 mole fraction water, and the distillate from column 2 is 0.4428 mole fraction water.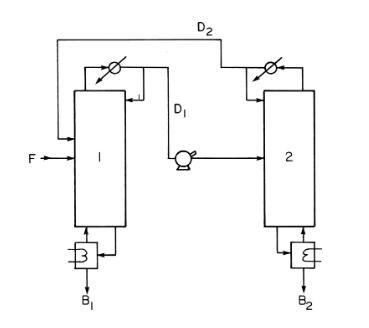
a. Find \(\mathrm{B}_{1}\) and \(\mathrm{B}_{2}\) flow rates in \(\mathrm{kmol} / \mathrm{h}\).
b. Find flow rates \(\mathrm{D}_{1}\) and \(\mathrm{D}_{2}\) in \(\mathrm{kmol} / \mathrm{h}\).
F D D2 2 B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


