Question
1. A column chromatography procedure for the separation of a polar and a non-polar compound calls for sequential elution with the following two solvents: methylene
1. A column chromatography procedure for the separation of a polar and a non-polar compound calls for sequential elution with the following two solvents: methylene chloride / hexanes
Which solvent should be used first?
a) methylene chloride
b) hexanes
c) it does not matter which solvent is used first
2. What happens if you load the spinach pigment mixture onto the column in too much methylene chloride?
a) the spinach pigments will not separate into individual components because they will travel rapidly down the column
b) the spinach pigments will evaporate
c) too much methylene chloride in the loading solvent will not affect the separation
3. Which of the following solvent mixtures is more polar?
a) ethyl acetate/hexanes 50:50
b) ethyl acetate/hexanes 80:20
4. We separated Carvone and limonene from spearmint oil, by micro column chromatography with silica gel and an eluotropic series of hexanes and acetone. If you start adding hexane, which compound, limonene or carvone, do you expect would elute first from the silica gel micro column?
Discuss the validity of the following statements:
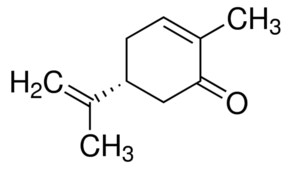
(R)-(-)-carvone
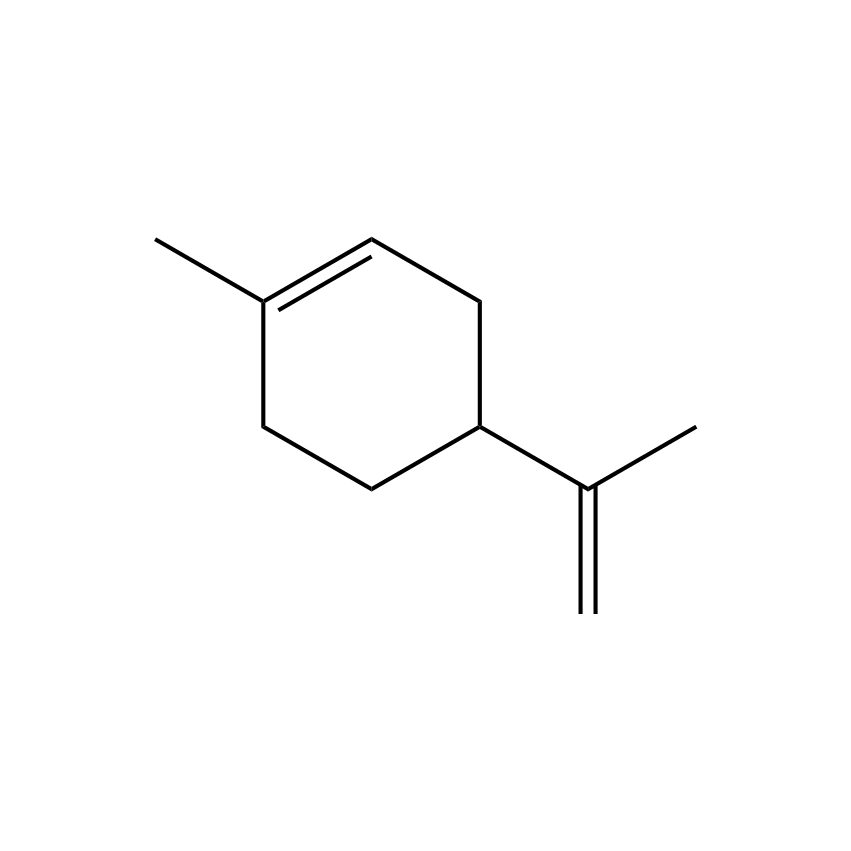
Limonene
a) A mixture of (+) - and (-)-carvone can be separated by column chromatography on silica gel using hexanes-acetone as elution solvent.
b) In general, column chromatography on silica gel is run with a series of solvents on increasing polarity.
c) Carvone is more polar than limonene
5. During the separation of limonene and carvone from spearmint oil we used a series ofsolvents in succession to run the column:
a) 2 mL hexanes
b) 2 mL hexanes
c) 2 mL hexanes
d) 2 mL 10% acetone in hexanes
e) 2mL 100%.1 acetone in hexanes
Briefly justify the elution order of limonene and carvone from the silica column.
6. The following two solvent systems were found to separate compounds X and Y by flash chromatography:
? X: hexanes/ethyl acetate 10:1
? Y: hexanes/ethyl acetate 10:4
Which is the more polar compound, X or Y?
7. In order to separate a mixture of X and Y as in problem (6) by column chromatography:
a) Which solvent system must be used first?
b) Which compound will elute from the column first (assuming you chose the correct solvent)?
8. A student loaded a mixture onto a small flash chromatography column in 1 mL of methylene chloride, then proceeded to elute with hexanes/ethyl acetate 10:1. He/she found that all of the mixture came off in the first 4 fractions, with no separation. What technical mistake did the student make?
9. You only see one compound coming off the column when you suspect two. Where might the other compound be? How can you recover this compound?
10. The students in a scheduled laboratory were going to separate compounds A, B, and C by column chromatography. The students constructed a column in the same way that you will do in experiment #9 and used silica gel as the stationary phase.
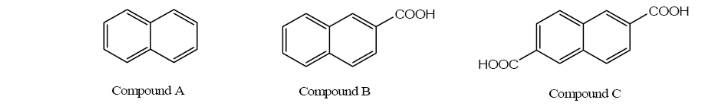
The students had three solvents available to elute the compounds from their columns:
100% hexane; 50/50, v/v hexane/ethyl acetate, and 100% ethyl acetate. In order to obtain complete separation of these three compounds they had to use all three
of the solvents.
a) Which solvent should be used first, which solvent should be used second, and which solvent should be used third? Explain your answer.
b) Which compound will elute from the column first, which compound will elute from the column second and which compound will elute from the column last?
Explain your answer.
11. Which kind of ultraviolet radiation contains the most energy? What is the range of wavelengths of this radiation?
12. Which form of ultraviolet radiation is most damaging to your eyes? What is the range of wavelengths for this radiation?
13. Describe the difference between absorbance and transmittance.
14. An absorbance spectrum shows a peak at 540 nm. What does this mean?
HC CH3 CH3 O
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Question1 The nonpolar compound is more soluble in hexanes while the polar compound is more soluble in methylene chloride Therefore the more polar compound will be retained longer on the column and el...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


