Suppose we have a volume of nitrogen plus a small amount of water vapor at (1.0 mathrm{~atm}).
Question:
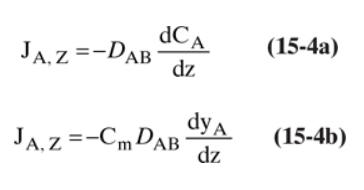
Suppose we have a volume of nitrogen plus a small amount of water vapor at \(1.0 \mathrm{~atm}\). The walls of the container are at \(25^{\circ} \mathrm{C}\), and there is a hot pipe at \(105^{\circ} \mathrm{C}\) running through the volume. Explain the behavior predicted by Eq. (15-4a), the behavior predicted by Eq. (15-4b), and the reasons that Eq. (15-4b) more closely predicts reality.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





