The system shown in Figure 3-9 is the stripping section of a distillation column with open steam
Question:
The system shown in Figure 3-9 is the stripping section of a distillation column with open steam heating. There is no condenser and no reboiler. This type of system is called a "beer still" in ethanol production. The beer is the fermentation broth consisting of water, ethanol, traces of other chemicals, and nonvolatile cell debris. Open steam heating is used because the cell debris will foul the heat exchange surface on a normal reboiler. The column is at \(1.0 \mathrm{~atm}\) and is adiabatic. The feed ethanol is \(10 \mathrm{~mol} \%\) ethanol \(\left(\mathrm{x}_{\mathrm{E}, \mathrm{F}}=0.10\right)\), is a saturated liquid, and feed flow rate \(\mathrm{F}=10 \mathrm{kmol} / \mathrm{h}\). The entering water vapor (aka steam) is pure water \(\left(\mathrm{y}_{\mathrm{E}, \mathrm{S}}=0\right)\), and the steam is saturated at \(1.0 \mathrm{~atm}\). The outlet bottoms liquid is \(\mathrm{x}_{\mathrm{E}, \mathrm{B}}=0.01\). The outlet distillate vapor is \(\mathrm{y}_{\mathrm{E}, \mathrm{D}}=0.40 \mathrm{~mol}\) fraction. Because stages in the column are equilibrium stages, the distillate is a saturated vapor and the bottoms is a saturated liquid. Boiling temperatures are in the ethanol-water VLE data in Table 2-1. Heat capacity and latent heat data are in Problem 2D9.
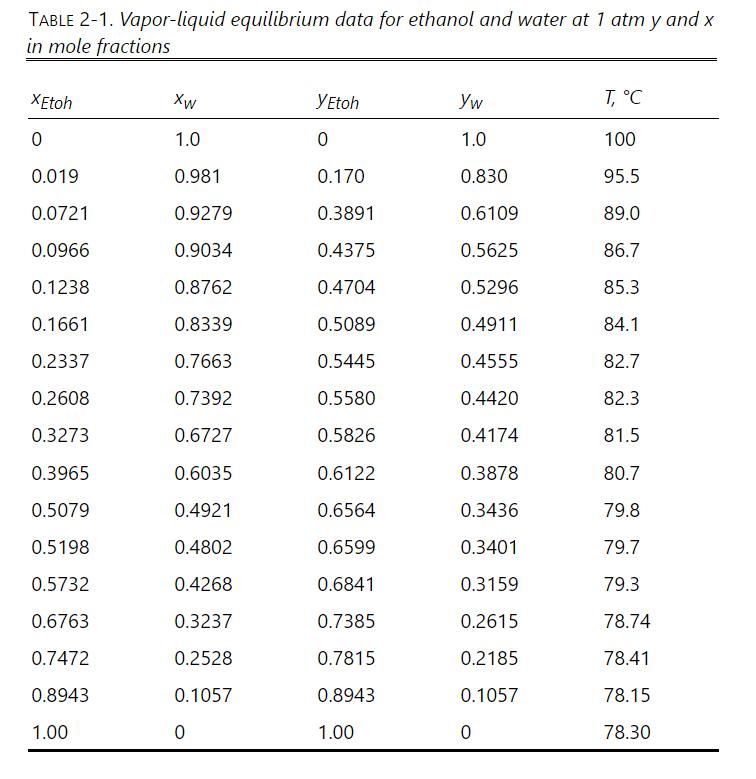
a. Solve the mass and energy balances to find \(S, B\), and \(D\). As a review of energy balances, use the data in problem 2D9 for part a, not Figure 2-4. Hint: Enthalpies of the vapor streams should be \(>10,000 \mathrm{kcal} / \mathrm{kmol}\), and enthalpies of the liquid streams should be \(
b. Check your enthalpies with Figure 2-4. Figure 2-4 is in weight, not mole units.
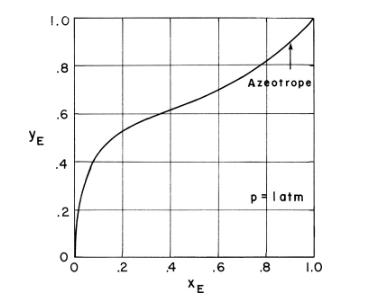
c. Determine the pressure of Figure 2-4 in atm.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





