This problem explores the azeotropic distillation column shown in Figure 8-18 with mole fraction profiles shown in
Question:
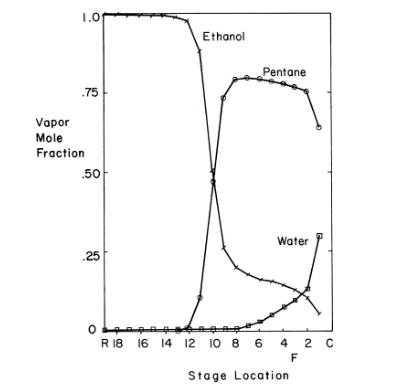
This problem explores the azeotropic distillation column shown in Figure 8-18 with mole fraction profiles shown in Figure 8-19. The feed to the distillation column in Figure 8 - 18 is a saturated liquid that is \(80.94 \mathrm{~mol} \%\) ethanol with the remainder being water. Do calculations on a basis of \(1000.0 \mathrm{kmol} / \mathrm{h}\). The ethanol product is \(99.8 \mathrm{~mol} \%\) ethanol and \(0.2 \mathrm{~mol} \%\) water. The water product is \(99.99 \mathrm{~mol} \%\) water and \(0.01 \mathrm{~mol} \%\) ethanol. In practice there will be traces of pentane in both products, but ignore this (assume makeup solvent \(=0\) ). The vapor mole fractions of the compounds leaving the top stage of the distillation column in Figure 8-18 (correspond to the profiles in Figure 8-19) are ethanol \(\mathrm{y}_{\mathrm{E}, 1}=0.0555\), water \(\mathrm{y}_{\mathrm{w}, 1}=\) 0.3000 , and pentane \(y_{p, 1}=0.6445\). Assume water and pentane are totally immiscible; thus, there is no water in the reflux to the distillation column, and there is no pentane in the feed to the stripping column. Ethanol distributes between the water and pentane layers in the settler. In the absence of data assume distribution coefficient

CMO is valid in both columns, and operation of the stripping column is at \(1.0 \mathrm{~atm}\).
Figure 8-18
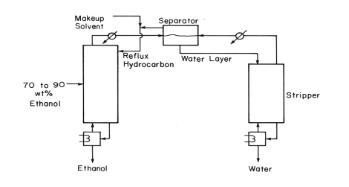
Figure 8-19
 a. Calculate the flow rates of ethanol product \(\mathrm{E}\) and water product \(\mathrm{W}\).
a. Calculate the flow rates of ethanol product \(\mathrm{E}\) and water product \(\mathrm{W}\).
b. What is \(\mathrm{V}_{1}\) in the distillation column, and what is the boilup rate in this column? (Note that \(\mathrm{Vy}_{\mathrm{w}, 1}\) must equal water in the feed minus the small amount of water lost in the ethanol product.) Also calculate \(\mathrm{L}_{0}\) \(=\) reflux rate.
c. Because no pentane leaves the bottom of the stripper, at steady state, all pentane that enters the settler must be returned to the distillation column in the reflux stream. In addition, all ethanol entering the settler minus the small amount of ethanol that leaves with the water product must be returned to the distillation column in the reflux stream. Calculate the steady-state values of \(\mathrm{x}_{\text {ethanol_in_pentane }}\) and \(\mathrm{x}_{\text {ethanol_in_water }}\) and the mole fraction ethanol in the reflux stream. Note that \(\mathrm{x}_{\text {ethanol_in_pentane }}\) and \(\mathrm{x}_{\text {ethanol_in_water }}\) are not equal to \(\mathrm{y}_{\mathrm{E}, 1}\).
d. If the boilup ratio in the stripper is 0.5 , what is the flow rate of the liquid fed to the stripper, and what is the flow rate of the vapor leaving the stripper? What is the mole fraction of ethanol in the vapor leaving the top stage of the stripper \(\left(\mathrm{y}_{\mathrm{E}, 1, \text { stripper }}\right)\) ?
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





