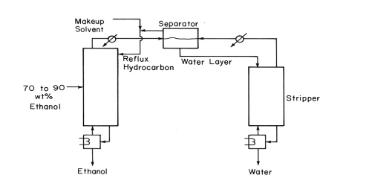
The azeotropic distillation shown in Figure 8-18 uses n-hexane as an entrainer to separate a feed that
Question:
The azeotropic distillation shown in Figure 8-18 uses n-hexane as an entrainer to separate a feed that is \(81.0 \mathrm{wt} \%\) ethanol and \(19.0 \mathrm{wt} \%\) water into ethanol and water. The saturated liquid feed is \(12,000 \mathrm{~kg} / \mathrm{h}\). The ethanol product is \(99.9 \mathrm{wt} \%\) ethanol, \(0.10 \mathrm{wt} \%\) hexane, and a trace of water. Water product is \(99.8 \mathrm{wt} \%\) water, \(0.20 \mathrm{wt} \%\) ethanol, and a trace of hexane. Do external mass balances, and calculate the flow rates of the makeup solvent ( \(\mathrm{n}\)-hexane), the ethanol product, and the water product. (Assume trace \(=0\). )
Figure 8-18
 Watch your decimal points when using weight fractions and \(\mathrm{wt} \%\).
Watch your decimal points when using weight fractions and \(\mathrm{wt} \%\).
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





