We are separating a mixture of benzene, toluene, and xylene in a distillation stripping column that has
Question:
We are separating a mixture of benzene, toluene, and xylene in a distillation stripping column that has a partial reboiler and no condenser. The feed is a saturated liquid, \(\mathrm{F}=135 \mathrm{kmol} / \mathrm{h}\), and feed is \(18.5 \mathrm{~mol} \%\) benzene, \(30.0 \mathrm{~mol} \%\) toluene, and remainder xylenes. Pressure is \(1.0 \mathrm{~atm}, \mathrm{CMO}\) is valid, and the relative volatilities are constant: \(\alpha_{\text {ben-tol }}=2.5, \alpha_{\text {tol-xy }}=3.030\). The boilup ratio »/B \(=5\), and the bottoms is 0.98 or higher mole fraction xylene. See problem 5.C1 for the method of calculating equilibrium with constant relative volatilities.
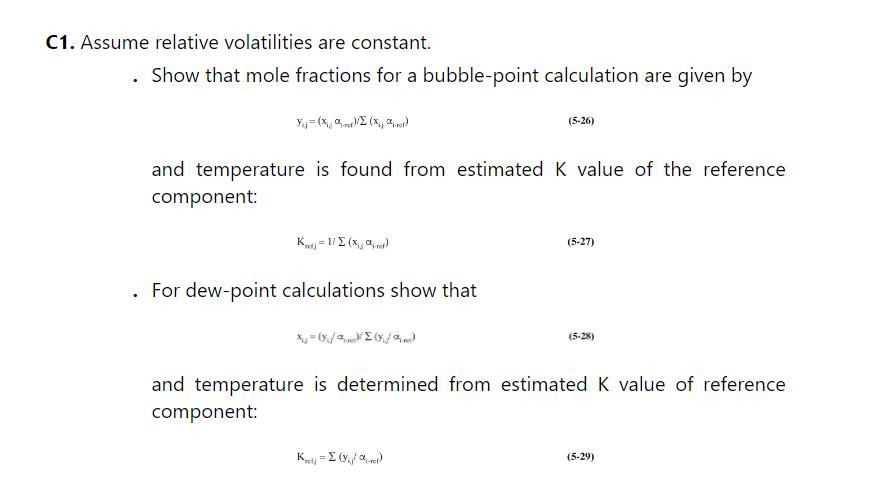
a. Based on the best assumption you can make, calculate B, mole fractions in bottoms, \(\mathrm{D}\), mole fractions in vapor distillate and \(\pi / \mathrm{r}\).
b. Show the calculation to determine mole fractions in liquid from the top stage (\#1) that are in equilibrium with the vapor distillate.
c. Show the calculation to determine mole fractions in vapor leaving stage 2. Stage-by-stage calculations are continued in problem 5.H1.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





