Question: Consider the ammonia process in which (mathrm{N}_{2}) and (mathrm{H}_{2}) (with impurities (mathrm{Ar}) and (mathrm{CH}_{4}) ) are converted to (mathrm{NH}_{3}) at high pressure (Figure 7.34). If
Consider the ammonia process in which \(\mathrm{N}_{2}\) and \(\mathrm{H}_{2}\) (with impurities \(\mathrm{Ar}\) and \(\mathrm{CH}_{4}\) ) are converted to \(\mathrm{NH}_{3}\) at high pressure (Figure 7.34). If using ASPEN PLUS, use the following subroutines:
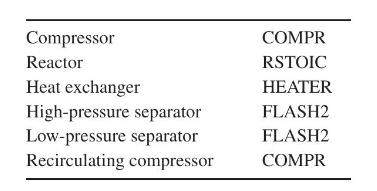
You are given the feed stream and fraction purged in the splitter. Prepare a simulation flowsheet and, when applicable, show the calculation sequence prepared by the process simulator (if using ASPEN PLUS, complete SEQUENCE USED WAS:).
Figure 7.34:-
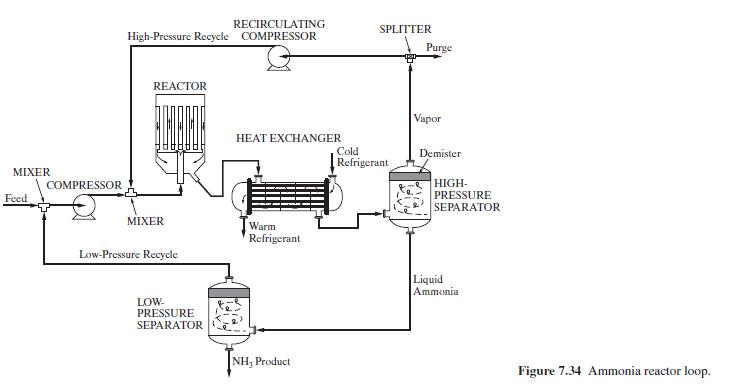
Compressor Reactor COMPR RSTOIC Heat exchanger HEATER High-pressure separator FLASH2 Low-pressure separator FLASH2 Recirculating compressor COMPR
Step by Step Solution
3.61 Rating (147 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


