Question: In the acrolein process introduced in problem 15.1, the catalyst is packed in tubes and the reactor is cooled using a circulating molten salt. The
In the acrolein process introduced in problem 15.1, the catalyst is packed in tubes and the reactor is cooled using a circulating molten salt. The molten salt rejects heat to boiling steam in a similar arrangement to that shown for phthalic anhydride in Figure 15.12. The required gas hourly space velocity is 200 standard litres (at STP)/litre.h. Design and size a reactor to produce 20 kt/y of acrolein.
Data from problem 15.1
Acrolein (H2C = CHCHO) is made by selective oxidation of propylene at 2 bar, 350°C using a molybdenum, iron and bismuth catalyst on a silica support. The reactor yields based on propylene are 85% acrolein, 10% acrylic acid and 5% light byproducts. The light byproducts are mostly acetaldehyde, but for the purpose of this problem it can be assumed that the yield is 85% acrolein and 15% acrylic acid. The feed to the reactor on a volume percent basis is propylene 6%, propane 28%, steam 6%, oxygen 11%, and balance nitrogen. Estimate the reactor cooling requirement for a plant that produces 20,000 metric tons per year (20 kt/y) of acrolein if the reactor is operated isothermally.
Figure 15.12
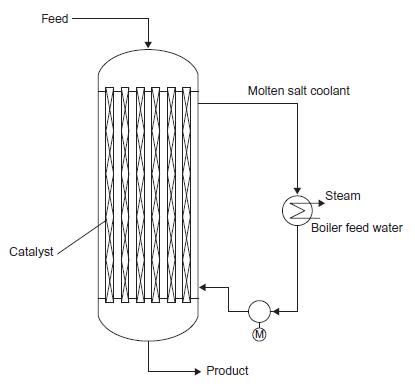
Feed Catalyst XXXX Molten salt coolant Product (M) Steam Boiler feed water
Step by Step Solution
3.43 Rating (150 Votes )
There are 3 Steps involved in it
The design and sizing of a reactor for the production of acrolein involves multiple steps including calculating the flow rates reactor volume and heat duty Ill provide guidance on how to approach this ... View full answer

Get step-by-step solutions from verified subject matter experts


