Question: In 2000, when the tPA process in Example 2.3 was synthesized using Chinese hamster ovary cells, data could not be located for the second reaction
In 2000, when the tPA process in Example 2.3 was synthesized using Chinese hamster ovary cells, data could not be located for the second reaction path using E-coli bacterial cells. Search the literature, especially the patents, since then to locate experimental results that give the:
(a) cell growth rate [cell/(mL-day)]
(b) tPA production rate [g/cell-day)]
(c) oxygen consumption rate \([\mathrm{mol} /(\) cell-hr)]
To check the patent literature, (Feasibility Study subsection). Can you locate data for other reaction paths?
Data From Example 2.3:-
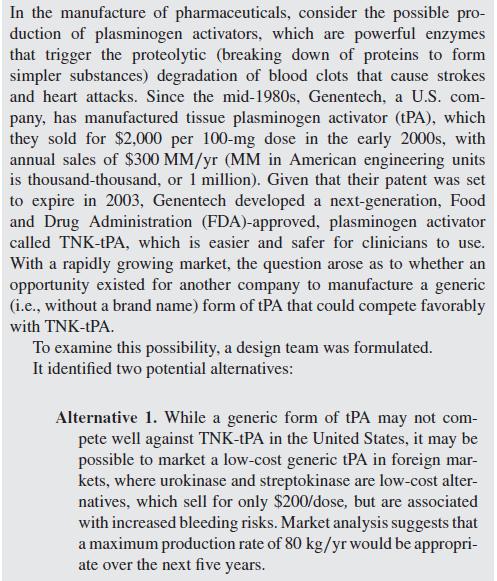
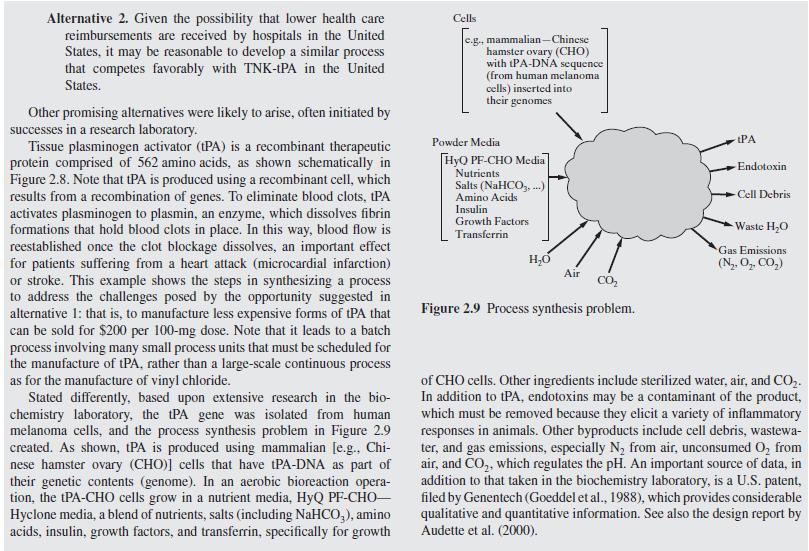
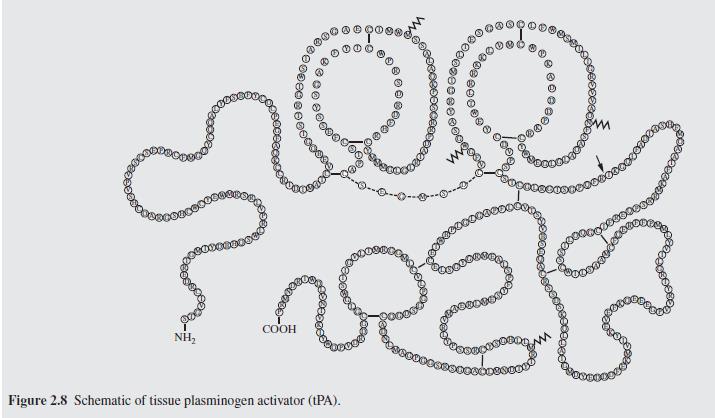
In the manufacture of pharmaceuticals, consider the possible pro- duction of plasminogen activators, which are powerful enzymes that trigger the proteolytic (breaking down of proteins to form simpler substances) degradation of blood clots that cause strokes and heart attacks. Since the mid-1980s, Genentech, a U.S. com- pany, has manufactured tissue plasminogen activator (tPA), which they sold for $2,000 per 100-mg dose in the early 2000s, with annual sales of $300 MM/yr (MM in American engineering units is thousand-thousand, or 1 million). Given that their patent was set to expire in 2003, Genentech developed a next-generation, Food and Drug Administration (FDA)-approved, plasminogen activator called TNK-tPA, which is easier and safer for clinicians to use. With a rapidly growing market, the question arose as to whether an opportunity existed for another company to manufacture a generic (i.e., without a brand name) form of tPA that could compete favorably with TNK-tPA. To examine this possibility, a design team was formulated. It identified two potential alternatives: Alternative 1. While a generic form of tPA may not com- pete well against TNK-tPA in the United States, it may be possible to market a low-cost generic tPA in foreign mar- kets, where urokinase and streptokinase are low-cost alter- natives, which sell for only $200/dose, but are associated with increased bleeding risks. Market analysis suggests that a maximum production rate of 80 kg/yr would be appropri- ate over the next five years.
Step by Step Solution
3.40 Rating (144 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


