Question: Revisit the helical flow reactor in Example 15.4. (a) Increase the mandrel radius to (50 mathrm{~cm}) and increase the volumetric flow rate to achieve the
Revisit the helical flow reactor in Example 15.4.
(a) Increase the mandrel radius to \(50 \mathrm{~cm}\) and increase the volumetric flow rate to achieve the same residence time. Determine the conversion of the reactants in one cycle of the helix. What is the effect of increasing the mandrel radius.
(b) Increase the pitch to \(10 \mathrm{~cm}\) and adjust the flow to achieve the same residence time. What is the effect of increasing the pitch?
Data From Example 15.4:-
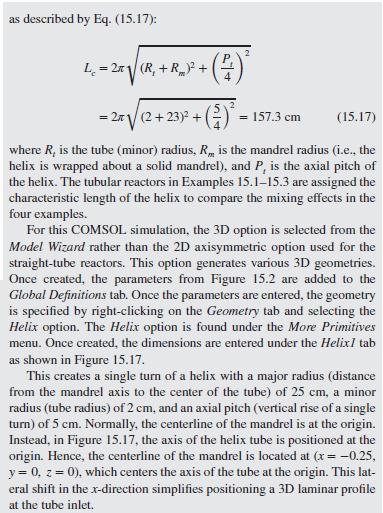
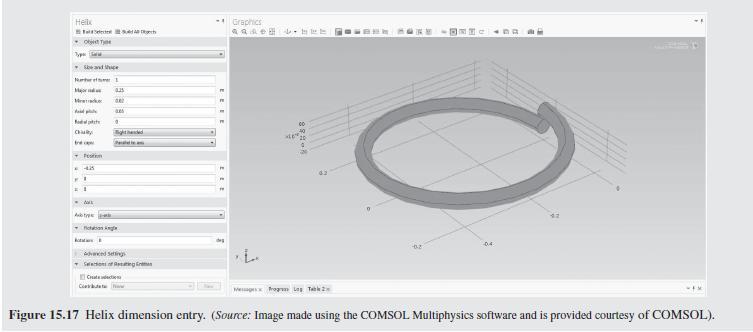
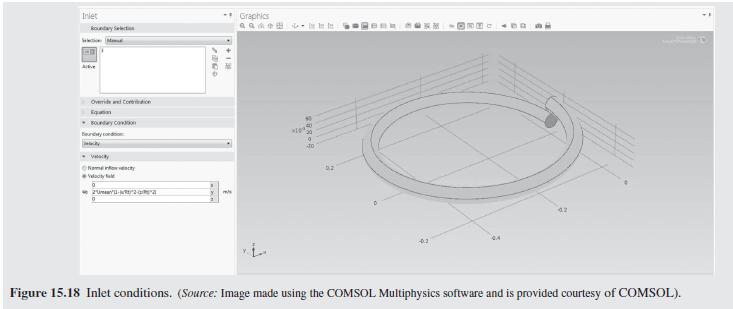
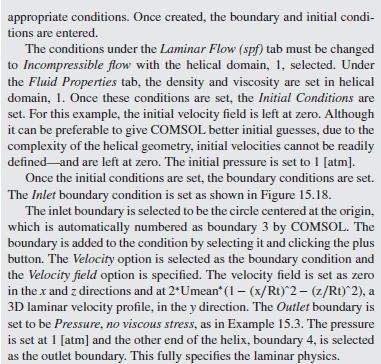
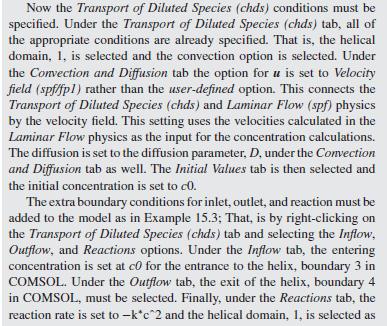
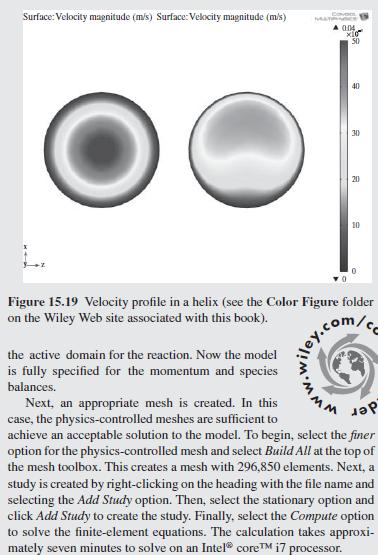

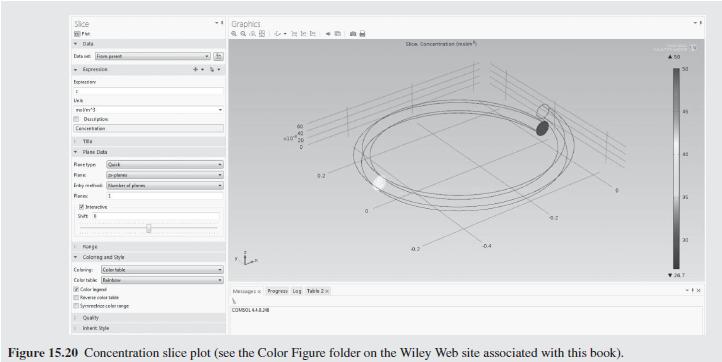

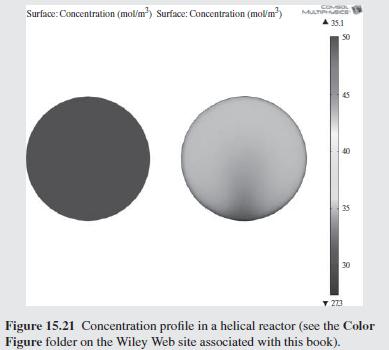
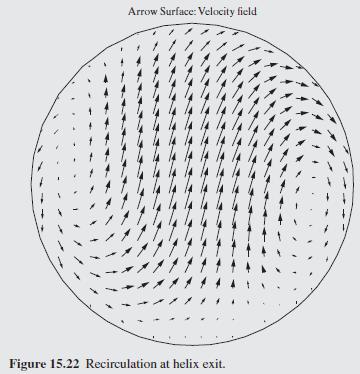
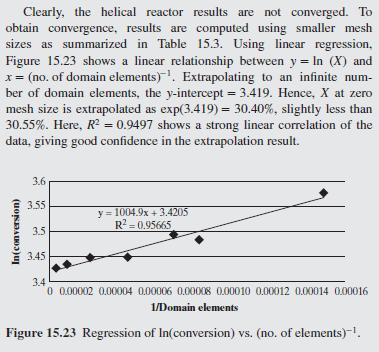
As a further example of how mixing impacts the conversion in a tubu- lar reactor, helical flow is examined (Slominski et al., 2011) for the following conditions: Feed at 0.05 mol/L of sodium hydroxide and 0.05 mol/L of ethyl acetate. Minor radius at 2 cm (0.7874 in) Major radius at 25 cm (9.8425 in) Pitch at 5 cm (1.9685 in) Temperature is 30C. Density of the reacting fluid (assumed to be pure water) is 996 kg/m and viscosity of the reacting fluid is 0.000798 Pa-s at 30C. Inlet volumetric flow rate of solution at 2 L/min (assumed to remain constant). Second-order irreversible reaction (first-order in each reactant) with rate constant, k = 8.9 L/mol-min at 30C.
Step by Step Solution
3.34 Rating (148 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


