A chemist needs to prepare a solution buffered at pH 4.30 using one of the following acids
Question:
A chemist needs to prepare a solution buffered at pH 4.30 using one of the following acids (and its sodium salt):
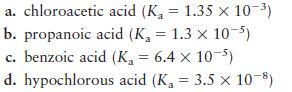
Calculate the ratio of [HA]/[A–] required for each system to yield a pH of 4.30. Which system will work best?
Transcribed Image Text:
a. chloroacetic acid (K = 1.35 x 10-) b. propanoic acid (K = 1.3 x 10-5) c. benzoic acid (K = 6.4 x 10-5) d. hypochlorous acid (K = 3.5 10-8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
A pH of 430 corresponds to H 10430 antilog430 50 x 105 M Since K values rather tha...View the full answer

Answered By

Muhammad Ghyas Asif
It is my obligation to present efficient services to my clients by providing a work of quality, unique, competent and relevant. I hope you have confidence in me and assign me the order and i promise to follow all the instructions and keep time.
4.60+
109+ Reviews
203+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A student is asked to prepare a buffer solution at pH = 8.60, using one of the following weak acids: HA (Ka = 2.7 10-3), HB (Ka = 4.4 10-6), HC (Ka = 2.6 10-9). Which acid should she choose? Why?
-
A solution contains a weak monoprotic acid HA and its sodium salt NaA both at 0.1 M concentration. Show that [OH2] = Kw/Ka.
-
Which acid would you choose to combine with its sodium salt to make a solution buffered at pH 4.25? For the best choice, calculate the ratio of the conjugate base to the acid required to attain the...
-
At the beginning of compression in a diesel cycle, T = 540 R, P = 30 lbf/in. 2 , and the state after combustion (heat addition) is 2600 R and 1000 lbf/in. 2 . Find the compression ratio, the thermal...
-
Compose the expression for a quantity whose dimension is length, using velocity of light c, mass of a particle m, and Planck's constant h. What is that quantity?
-
A financial institution has entered into an interest rate swap with company X. Under the terms of the swap, it receives 10% per annum and pays six-month LIBOR on a principal of $10 million for five...
-
List the typical steps in the control process. (pp. 428429)
-
Starbucks Corporationlike all other businessesmakes adjusting en-tries at year- end in order to measure assets, liabilities, revenues, and expenses properly. Examine Starbucks Corporations Balance...
-
Read the case situation given below and answer the following questions. Pearl Continental Hotel or PC Hotel is an elegant 5 Star Hotel in the central downtown of Karachi. The hotel offers all...
-
Hydrogen cyanide gas (HCN) is a powerful respiratory inhibitor that is highly toxic. It is a very weak acid (K a = 6.2 10 10 ) when dissolved in water. If a 50.0-mL sample of 0.100 M HCN is...
-
Calculate the change in pH that occurs when 0.010 mol of gaseous HCl is added to 1.0 L of each of the following solutions. Solution A: 5.00 M HC 2 H 3 O 2 and 5.00 M NaC 2 H 3 O 2 Solution B: 0.050 M...
-
Use the following adjusted trial balance of Hanson Trucking Company to prepare a classified balance sheet as of December 31, 2023. Account Title Cash Accounts receivable Office supplies Trucks...
-
Machine cost = $15,000; life = 8 years; salvage value = $3,000. What minimum cash return would an investor demand annually from the operation of this machine if he desires interest annually at the...
-
Write a program that prompts for the student's name, the number of exams, the exam score of each exam, and display the letter grade for the student. Read the entire problem description before coding....
-
Considering only the vertical stabilizer and rudder, explain the aerodynamic forces and moments that are created. You must include at least applicable airfoil terminology, description of force...
-
part. Review A bicycle wheel is rotating at 47 rpm when the cyclist begins to pedal harder, giving the wheel a constant angular acceleration of 0.44 rad/s. Part B How many revolutions does the wheel...
-
Suppose the number of students who register for a certain class each semester can be modeled by a Poisson distribution with average 10. Suppose further that each student passes the class with...
-
What are the major factors influencing the alignment of internal strategies to external strategies?
-
Doorharmony Company makes doorbells. It has a weighted- average cost of capital of 5% and total assets of $ 5,900,000. Doorharmony has current liabilities of $ 750,000. Its operating income for the...
-
Estimate the value of the quantum number n for the uppermost filled level in a one-dimensional line of copper atoms of length 1.0 mm.
-
What is the difference between homogeneous and heterogeneous alloys? Give examples of each type.
-
For which of the following molecules will dipoledipole interactions be important: (a) O 2 ; (b) O 3 ; (c) CO 2 ; (d) SO 2 ?
-
The tolal landed coet with the order gaantly sire of 6,000 unts is 4 (Enter your response roundod to the nearest dolar)
-
Boyne Inc. had beginning inventory of $12,000 at cost and $20,000 at retail. Net purchases were $120,000 at cost and $170,000 at retail. Net markups were $10,000, net markdowns were $7,000, and sales...
-
Apple inc. CONDENSED CONSOLIDATED BALANCE SHEETS (Unaudited) (In milions, except number of shares which are reflected in thousands and par value) LABILITES AND SHAREHOLDERS' EQUITY: Current...

Study smarter with the SolutionInn App


