(a) Consider the substances shown in parts (a), (c), and (d) in the second illustration in Box...
Question:
(a) Consider the substances shown in parts (a), (c), and (d) in the second illustration in Box 8F.1.
(b) In which of these three parts of the figure will the interactions be the strongest?
(c) In which will they be the weakest?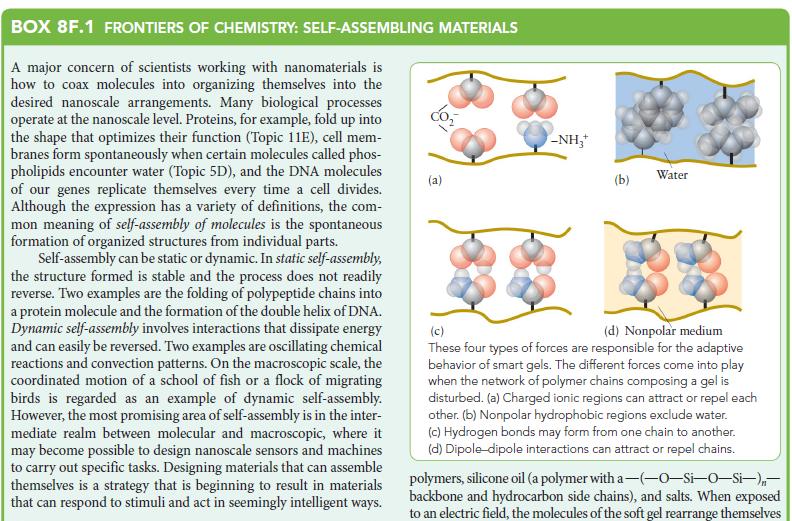

Transcribed Image Text:
BOX 8F.1 FRONTIERS OF CHEMISTRY: SELF-ASSEMBLING MATERIALS A major concern of scientists working with nanomaterials is how to coax molecules into organizing themselves into the desired nanoscale arrangements. Many biological processes operate at the nanoscale level. Proteins, for example, fold up into the shape that optimizes their function (Topic 11E), cell mem- branes form spontaneously when certain molecules called phos- pholipids encounter water (Topic 5D), and the DNA molecules of our genes replicate themselves every time a cell divides. Although the expression has a variety of definitions, the com- mon meaning of self-assembly of molecules is the spontaneous formation of organized structures from individual parts. Self-assembly can be static or dynamic. In static self-assembly, the structure formed is stable and the process does not readily reverse. Two examples are the folding of polypeptide chains into a protein molecule and the formation of the double helix of DNA. Dynamic self-assembly involves interactions that dissipate energy and can easily be reversed. Two examples are oscillating chemical reactions and convection patterns. On the macroscopic scale, the coordinated motion of a school of fish or a flock of migrating birds is regarded as an example of dynamic self-assembly. However, the most promising area of self-assembly is in the inter- mediate realm between molecular and macroscopic, where it may become possible to design nanoscale sensors and machines to carry out specific tasks. Designing materials that can assemble themselves is a strategy that is beginning to result in materials that can respond to stimuli and act in seemingly intelligent ways. -NH₂* (b) Water (c) (d) Nonpolar medium These four types of forces are responsible for the adaptive behavior of smart gels. The different forces come into play when the network of polymer chains composing a gel is disturbed. (a) Charged ionic regions can attract or repel each other. (b) Nonpolar hydrophobic regions exclude water. (c) Hydrogen bonds may form from one chain to another. (d) Dipole-dipole interactions can attract or repel chains. polymers, silicone oil (a polymer with a-(-O-Si-O-Si—),— backbone and hydrocarbon side chains), and salts. When exposed to an electric field, the molecules of the soft gel rearrange themselves
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Ionion forces are among the strongest intermolecula...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The Hampshire Company manufactures umbrellas that sell for $12.50 each. In 2014, the company made and sold 60,000 umbrellas. The company had fixed manufacturing costs of $216,000. It also had fixed...
-
Three college seniors with majors in accounting are discussing alternative career plans. All three want to enter careers that will help to ensure the integrity of financial reporting. The first wants...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A company had a broken printer which they deemed was not worth fixing and thus discarded it. The original cost of the printer was $7,500 and the accumulated depreciation at the time of disposal was...
-
Suppose that you had created an OS facility that implemented monitors, but not condition variables. Show how to implement condition variables using Dijkstra semaphores.
-
Comprehensive problem; ABC manufacturing two products. Dinettes Inc. operates at capacity and 2es glass topped dining tables and wooden chairs, which are then typically sold as sets of four chairs...
-
A light ray reflects from a mirror hanging vertically on a wall in your bathroom, and the ray strikes a particular point on the opposite wall (Fig. P24.6). The bottom end of the mirror is then tilted...
-
A 500-W heating coil designed to operate from 110 V is made of Nichrome wire 0.500 mm in diameter. (a) Assuming that the resistivity of the Nichrome remains constant at its 20.0C value, find the...
-
Please help me out, I need this as soon as possible, take your time but please answer when you can. Journal entry worksheet Record the year-end adjustment to fair value, if any. Note: Enter debits...
-
Methanol, CH 3 OH, is a clean-burning liquid fuel being used as a replacement for gasoline. Calculate the theoretical yield in kilograms of CO 2 produced by the combustion of 1.00 L of methanol (of...
-
The gas NO is released to the stratosphere by jet engines. Because it can contribute to the destruction of stratospheric ozone, its concentration is monitored closely. One monitoring technique...
-
What is the rhizosphere. and in what ways does the soil in the rhizosphere differ from the rest of the soil?
-
Small town Diners has a policy of treating dividends as a passive residual. It forecasts that net earnings after taxes in the coming year will be $500,000. The firm has earned the same $500,000 for...
-
Part 1-Chi-Square Goodness-of-Fit Tests A health psychologist was interested in women's workout preferences. Of the 56 participants surveyed, 22 preferred running, 8 preferred swimming, 15 preferred...
-
The Campbell Company is considering adding a robotic paint sprayer to its production line. The sprayer's base price is $1,070,000, and it would cost another $21,000 to install it. The machine falls...
-
Problem 1. (10 points) Consider the space X = R22 and the map L XX defined as traceX -traceX L:X X = X 0 0 1. Show that L is a linear map; 2. Find the matrix representation M = mat L in the canonical...
-
Suppose that the exchange rate is 1.25 = 1.00. Options (calls and puts) are available on the Philadelphia exchangein units of10,000 with strike prices of $1.60/1.00. Options (calls and puts) are...
-
(i) Estimate equation (10.2) using all the data in PHILLIPS.RAW and report the results in the usual form. How many observations do you have now? (ii) Compare the estimates from part (i) with those in...
-
A test car is driven a fixed distance of n miles along a straight highway. (Here n Z+.) The car travels at one mile per hour for the first mile, two miles per hour for the second mile, four miles...
-
Consider the 25 players on a professional baseball team. At any point, nine players are on the field. a. How many nine-player batting orders are possible given that the order of batting is important?...
-
Imagine an experiment in which you flip a coin four times. Furthermore, the coin is balanced fairly such that the probability of landing heads or tails is equivalent. After tossing the coin 10 times,...
-
In Chapter 34, we will model particle diffusion as a random walk in one dimension. In such processes, the probability of moving an individual step in the +x or x direction is equal to one half....
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


