The gas NO is released to the stratosphere by jet engines. Because it can contribute to the
Question:
The gas NO is released to the stratosphere by jet engines. Because it can contribute to the destruction of stratospheric ozone, its concentration is monitored closely. One monitoring technique measures the chemiluminescence given off by the reaction NO(g) + O3(g) → NO2*(g) + O2(g). The asterisk indicates that NO2 is produced in an excited state. The excited NO2 molecule gives off red light and infrared radiation as it returns to the ground state. Because both NO and NO2 may be present in the air sample, part of the sample is exposed to a reducing agent, which converts all the NO2 to NO. Two samples are then analyzed, first air from the unreduced original sample, which gives the concentration of NO, then air from the reduced sample. The concentration of NO2 in the air is the difference between the two measurements. In one experiment, two samples were collected, one from a cloud and one from clear air. The following measurements were made at 9 km above the Pacific Ocean (ppt denotes parts per trillion, 1 part per 1012, by molecules):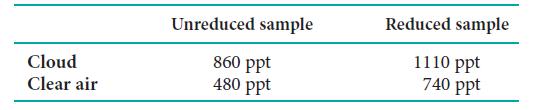
(a) Determine the concentrations (in ppt) of NO and NO2 in each air sample.
(b) In which part of the atmosphere is the concentration of NO greater?
(c) Explain the difference, taking into account other sources of NO in the atmosphere besides airplanes.
(d) Some of the NO molecules released by jet engines react with the hydroxyl radical, ·OH. Draw the Lewis structure of the most likely product and state its name.
(e) Is the product of the reaction in part (d) likely to be more stable than NO? Explain your reasoning.
(f) In the stratosphere, NO catalyzes the conversion of O3 to O2 in a two-step reaction with the intermediate NO2. The overall equation for the reaction is O3(g) + O(g) → 2 O2(g) and the rate law for the reaction is Rate = kr[NO][O3]. Write an acceptable two-step mechanism for the reaction, indicating which step is the slow step.
(g) At 230°C, the temperature of the air from which the samples were taken, the rate constant for the reaction in part (f) is 6 * 10–15 cm3 · molecule–1 · s–1. If the concentration of ozone in the air from which the NO clear air sample was taken was 480 ppt and the total pressure of the air was 220 mbar, what was the rate at which ozone was being destroyed in the air at 230°C?
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





