The tiny structures such as spheres and tubes formed by carbon atoms are the basis for a
Question:
The tiny structures such as spheres and tubes formed by carbon atoms are the basis for a large part of the field of nanotechnology. Boron nitride forms similar structures.
(a) What is the hybridization of carbon atoms in carbon nanotubes and of boron and nitrogen atoms in boron nitride nanotubes?
(b) A simple carbon nanotube is made up of a sheet resembling a layer of graphene (similar to chicken wire) that has been curled up and bonded to itself. How many hexagons must be strung together (the hexagons denoted by the colored bonds in the following diagram) around the circumference of a nanotube with the “armchair” configuration to form a tube that is approximately 1.3 nm in diameter? The diagram shows the orientation of hexagons with respect to the curvature of the nanotube; a representative nanotube is shown for reference. The C—C bond distance in carbon nanotubes is 142 pm.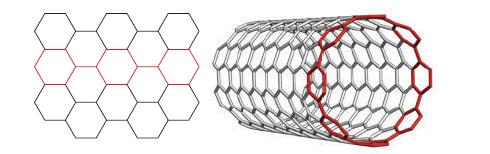
(c) Buckminsterfullerene, C60, can be hydrogenated, but, a compound with the formula C60H60 has not yet been prepared. The most hydrogenated form of C60 known is C60H36. Why does hydrogenation stop at this point?
(d) Boron nitride can form nanotubes, but it does not form spheres that resemble buckminsterfullerene. Examine the structure of buckminsterfullerene and suggest a reason why BN cannot form spheres.
(e) In its cubic crystalline form the nitrogen atoms of BN form a face-centered cubic unit cell in which half the tetrahedral interstitial sites are occupied by B atoms (if all B and N atoms were replaced by C atoms, the diamond structure would result). Calculate the density of cubic BN if the edge length of the unit cell is 361.5 pm.
(f) The density of hexagonal BN is 2.29 g · cm–3. Which form of BN is favored at high pressures, hexagonal or cubic?
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





