You are working in the chemical stockroom of a university, and a teaching assistant requests two inorganic
Question:
You are working in the chemical stockroom of a university, and a teaching assistant requests two inorganic reagents. You want to make sure you deliver the correct materials, so you need to compare both the formulas and names from the request with the labels on the bottles.
(a) Name the coordination compound [Co(NH3)3(OH2)3]2(SO4)3.
(b) Write the formula of sodium dichloridobis(oxalato)platinate(IV).
PLAN Apply the rules in Toolbox 9C.1.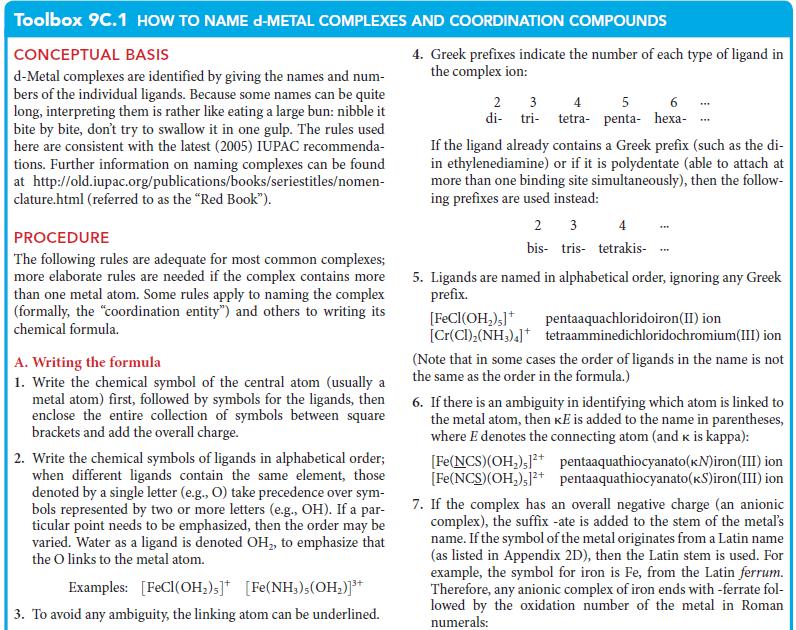
![Examples: [Fe (NCS) (OH),]+ [Fe (NCS) (OH),]+ B. Naming the complex 1. Name the ligands first, and then the](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/1/0/1/853659287ddc8fe91704101852651.jpg)
Transcribed Image Text:
Toolbox 9C.1 HOW TO NAME D-METAL COMPLEXES AND COORDINATION COMPOUNDS CONCEPTUAL BASIS d-Metal complexes are identified by giving the names and num- bers of the individual ligands. Because some names can be quite long, interpreting them is rather like eating a large bun: nibble it bite by bite, don't try to swallow it in one gulp. The rules used here are consistent with the latest (2005) IUPAC recommenda- tions. Further information on naming complexes can be found at http://old.iupac.org/publications/books/seriestitles/nomen- clature.html (referred to as the "Red Book"). PROCEDURE The following rules are adequate for most common complexes; more elaborate rules are needed if the complex contains more than one metal atom. Some rules apply to naming the complex (formally, the "coordination entity") and others to writing its chemical formula. A. Writing the formula 1. Write the chemical symbol of the central atom (usually a metal atom) first, followed by symbols for the ligands, then enclose the entire collection of symbols between square brackets and add the overall charge. 2. Write the chemical symbols of ligands in alphabetical order; when different ligands contain the same element, those denoted by a single letter (e.g., O) take precedence over sym- bols represented by two or more letters (e.g., OH). If a par- ticular point needs to be emphasized, then the order may be varied. Water as a ligand is denoted OH₂, to emphasize that the O links to the metal atom. Examples: [FeCl(OH₂)s] [Fe(NH₂),(OH₂)]³+ 3. To avoid any ambiguity, the linking atom can be underlined. 4. Greek prefixes indicate the number of each type of ligand in the complex ion: 2 3 5 di-tri- tetra- penta- hexa- If the ligand already contains a Greek prefix (such as the di- in ethylenediamine) or if it is polydentate (able to attach at more than one binding site simultaneously), then the follow- ing prefixes are used instead: 2 3 4 bis tris tetrakis- [FeCl(OH₂),]+ [Cr(CI),(NH3)4] *** 5. Ligands are named in alphabetical order, ignoring any Greek prefix. pentaaquachloridoiron(II) ion tetraamminedichloridochromium(III) ion (Note that in some cases the order of ligands in the name is not the same as the order in the formula.) 6. If there is an ambiguity in identifying which atom is linked to the metal atom, then KE is added to the name in parentheses, where E denotes the connecting atom (and k is kappa): [Fe(NCS)(OH₂)]²+ pentaaquathiocyanato (kN)iron(III) ion [Fe(NCS)(OH₂),12+ pentaaquathiocyanato(KS)iron(III) ion 7. If the complex has an overall negative charge (an anionic complex), the suffix -ate is added to the stem of the metal's name. If the symbol of the metal originates from a Latin name (as listed in Appendix 2D), then the Latin stem is used. For example, the symbol for iron is Fe, from the Latin ferrum. Therefore, any anionic complex of iron ends with -ferrate fol- lowed by the oxidation number of the metal in Roman numerals:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a There are three SO ions for every two complex ions The complex cation must have a charge of 3 CoNH...View the full answer

Answered By

Dorcas Juliet
I am a proficient tutor and writer with over 4 years experience, I can deliver A+ works in all fields related to business and economics subject. Kindly hire me for excellent papers
4.70+
10+ Reviews
51+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Fifty clients of an outpatient mental clinic take an anxiety inventory. Scores range from 1 to 10. Here are the scores. a. Counting up from the bottom of the table, what is the ballpark median...
-
Elysian Fields, Inc., uses a maximum payback period of 6 years and currently must choose between two mutually exclusive projects. Project Hydrogen requires ar initial outlay of $25,000; project...
-
You are working in the office of the vice president of administration at International Telecon (IT) as a senior financial planner. IT is a Fortune 500 firm with sales approaching $ 1 billion. IT...
-
Perpetual inventory using FIFO Beginning inventory, purchases, and sales for Item Zeta9 are as follows: Oct. 1 Inventory 37 units @ $19 Oct. 7 Sale Oct. 15 Purchase Oct. 24 Sale Assuming a perpetual...
-
Suppose processes P 0 and P 1 share variable V 2 , processes P 1 and P 2 share variable V 0 , and processes P 2 and P 3 share variable V 1 . In addition, P 0 , P 1 , and P 2 run concurrently. Write a...
-
A vertical force T is exerted on a 5-kg body near the surface of the earth, as shown in Figure. Find the acceleration of the body if (a) T = 5 N,? (b) T = 10 N, (c) T = 100N. 5kg
-
A plane mirror made of a very thin piece of glass lies flat on the ground. As shown in Figure P24.10, one end of the mirror is 2.0 m from you and the other end is 30 m from a nearby tree. You are 1.8...
-
Diekmann Company, a U.S.-based company, acquired a 100 percent interest in Rakona A.S. in the Czech Republic on January 1, 2016, when the exchange rate for the Czech koruna (Ks) was $0.05. Rakona's...
-
Cost of Units Transferred Out and Ending Work in Process The costs per equivalent unit of direct materials and conversion in the Rolling Department of Oak Ridge Steel Company are $1.55 and $2.30,...
-
The tiny structures such as spheres and tubes formed by carbon atoms are the basis for a large part of the field of nanotechnology. Boron nitride forms similar structures. (a)What is the...
-
The compound Cr(OH) 3 is very insoluble in water; therefore, electrochemical methods must be used to determine its K sp . Given that the reduction of Cr(OH) 3 (s) to Cr(s) and hydroxide ions has a...
-
Consider the equilibrium stage shown in. Conduct a degrees-of-freedom analysis by performing the following steps:(a) List and count the variables.(b) Write and count the equations relating the...
-
The figure shows a turbine-driven pump that provides water, at high pressure, to a tank located 25-m higher than the pump. Steady-state operating data for the turbine and the pump are labelled on the...
-
Step 1 Step 2 1. Sketch what step 4 and then step 5 would look like. Step 4 Step S 2. How many black triangles are in each step? Step 1 black A = | Step 2 = 4 black A's step 3 = 13 black D's 3. What...
-
The pressure cooker pictured here consists of a light pressure vessel with a heavy lid of weight W. When the lid is secured, the vessel is filled with a hot pressurized gas of pressure p. After some...
-
5) A large group of students took a test in Finite Math where the grades had a mean of 72 and a standard deviation of 4. Assume that the distribution of these grades is approximated by a normal...
-
Q9 (5 points) According to Dr. Henry Mintzberg, a noted management scholar from McGill University in Montreal, PQ, "business organizations perform only two activities of consequence." What are these...
-
Decide if you agree or disagree with each of the following statements and give a brief explanation of your decision: (i) Like cross-sectional observations, we can assume that most time series...
-
On average there are four traffic accidents in a city during one hour of rush-hour traffic. Use the Poisson distribution to calculate the probability that in one such hour there arc (a) No accidents...
-
Assume that the probability of occupying a given energy state is given by the relationship provided in Problem P29.27. In problem 29.27 In a subsequent chapter we will encounter the energy...
-
Consider the following probability distribution corresponding to a particle located between point x = 0 and x = a: a. Determine the normalization constant, C. b. Determine ©xª. c. Determine...
-
Consider the probability distribution for molecular velocities in one dimension (v x ) given by a. Determine the normalization constant, C. b. Determine ©v x ª. c. Determine ©v 2 x...
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...

Study smarter with the SolutionInn App


