(a) From the data in Appendix 2A, calculate the enthalpy required to vaporize 1 mol CH 3...
Question:
(a) From the data in Appendix 2A, calculate the enthalpy required to vaporize 1 mol CH3OH(l) at 298.2 K.
(b) Given that the molar heat capacity, CP,m, of liquid methanol is 81.6 J · K–1 · mol–1 and that of gaseous methanol is 43.89 J · K–1 · mol–1, calculate the enthalpy of vaporization of methanol at its boiling point (64.7°C).
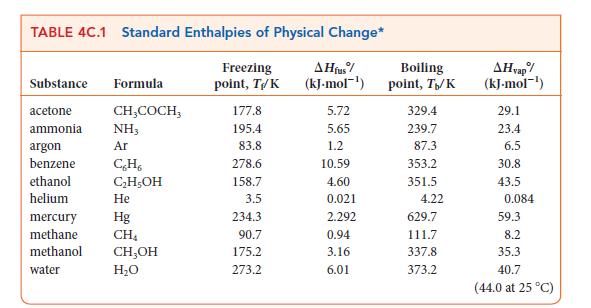
(c) Compare the value obtained in part (b) with that found in Table 4C.1. What is the source of difference between these values?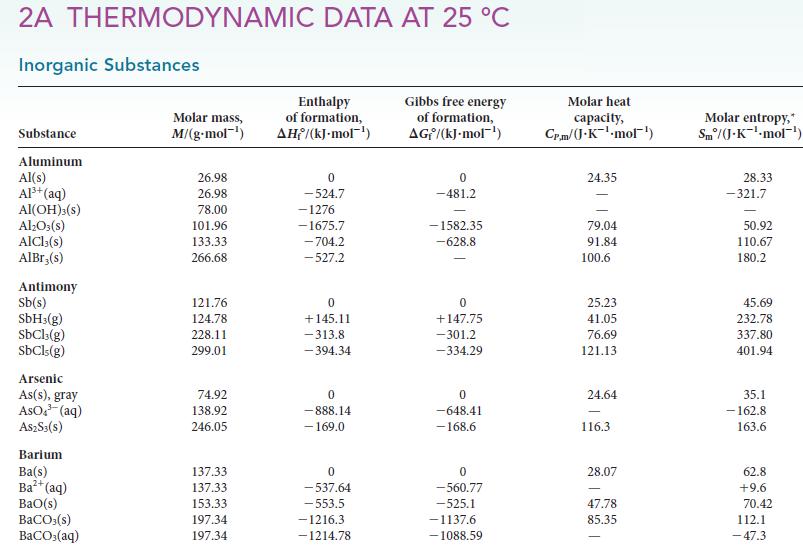
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





