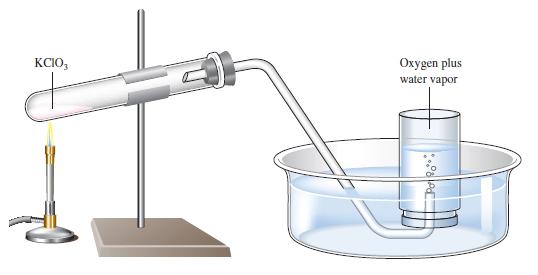
A sample of solid potassium chlorate (KClO 3 ) was heated in a test tube (see Fig.
Question:
A sample of solid potassium chlorate (KClO3) was heated in a test tube (see Fig. 5.10) and decomposed according to the following reaction:
![]()
The oxygen produced was collected by displacement of water at 22°C at a total pressure of 754 torr. The volume of the gas collected was 0.650 L, and the vapor pressure of water at 22°C is 21 torr. Calculate the partial pressure of O2 in the gas collected and the mass of KClO3 in the sample that was decomposed.
Figure 5.10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





