A solution was prepared by dissolving 18.00 g of glucose in 150.0 g of water. The resulting
Question:
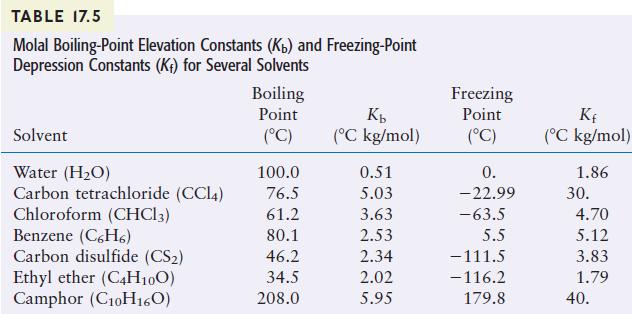
A solution was prepared by dissolving 18.00 g of glucose in 150.0 g of water. The resulting solution was found to have a boiling point of 100.34°C at 1 atm. Calculate the molar mass of glucose. Glucose is a molecular solid that is present as individual molecules in solution.
Transcribed Image Text:
TABLE 17.5 Molal Boiling-Point Elevation Constants (Kb) and Freezing-Point Depression Constants (K) for Several Solvents Solvent Water (HO) Carbon tetrachloride (CC14) Chloroform (CHC13) Benzene (C6H6) Carbon disulfide (CS) Ethyl ether (C4H10O) Camphor (C10H160) Boiling Point (C) 100.0 76.5 61.2 80.1 46.2 34.5 208.0 Kb (C kg/mol) 0.51 5.03 3.63 2.53 2.34 2.02 5.95 Freezing Point (C) 0. -22.99 -63.5 5.5 -111.5 -116.2 179.8 Kf (C kg/mol) 1.86 30. 4.70 5.12 3.83 1.79 40.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
We make use of the equation AT Kumsolute AT 10034C 10000C 034...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A solid is generated by revolving about the x-axis the region in the first quadrant bounded by the coordinate axes, linex = and graph of y = 1+ sin x. Which of the following integrals below will...
-
A solution was prepared by dissolving 0.834 g of sulfur, S8, in 100.0 g of acetic acid, HC2H3O2. Calculate the freezing point and boiling point of the solution.
-
A solution was prepared by dissolving 5.76 g of KCl MgCl2 6H2O (277.85 g/mol) in sufficient water to give 2.000 L. Calculate (a) The molar analytical concentration of KCl MgCl2 in this solution....
-
What is the best way to describe automation? developing highly advanced robots that can mimic higher-levelhuman thinking making human workers fully reliant on technology to performtheir job...
-
The table gives the population of the United States, in millions, for the years 19002000. (a) Use the exponential model and the census figures for 1900 and 1910 to predict the population in 2000....
-
Selected financial data (in millions) of two intense competitors in a recent year are presented here: \section*{Instructions} For each company, compute these values and ratios: (a) Working capital...
-
In regression, what is an error of prediction? LO9
-
Cal Murphy is emigrating from Canada to take up residence in Jakarta, Indonesia. At the time of his departure, Cal will have the following Canadian assets: Cal has been impressed by the capital...
-
Question 4 (Definition and Mathematical Explanation) (2.5%) a) Explain what a fixed exchange rate is and give numerical example using values. b) Explain what a floating exchange rate is and give...
-
What mass of ethylene glycol (C 2 H 6 O 2 ), the main component of antifreeze, must be added to 10.0 L of water to produce a solution for use in a cars radiator that freezes at 10.0F (23.3C)? Assume...
-
A solution was prepared by adding 20.0 g of urea to 125 g of water at 25C, a temperature at which pure water has a vapor pressure of 23.76 torr. The observed vapor pressure of the solution was found...
-
For the poll described in the Chapter Problem, assume that the respondents had been asked for their political party affiliation, and the responses were coded as 0 (for Democrat), 1 (for Republican),...
-
1. (5 pts) Given y[n]= 2y[n-1] and y[0]=2, Write MATLAB code to calculate and plot y for 0
-
F ( t ) = t 4 + 1 8 t 2 + 8 1 2 , g ( t ) = ( t + 3 ) / 3 ; find ( f o g ) ( 9 )
-
How did they calculate allocated cost FLIGHT A FLIGHT 350 615 FLIGHT 3 1 Go GALS 20 G EXISTING SCHEME, DETERMINE THE OVE OR FLIGHTS A, B, AND C. 2 ED AT 7.00 PER K1.00 OF PILOT SALAF TOTAL NON-SALARY...
-
High Tech ManufacturingInc., incurred total indirect manufacturing labor costs of $540,000. The company is labor-intensive. Total labor hours during the period were 5,000. Using qualitativeanalysis,...
-
Start with AS/AD and IS/MP in full employment equilibrium. Assume the is a massive positive aggregate demand shock. How would this affect AS/AD and IS/MP and prices and output relative to the full...
-
The model in Section 5.5, Exercise 23. For the above problem, add direction arrows to the phase-plane.
-
Answer the following two independent questions. a. MM Corporation is considering several proposed investments for the coming budget year. MM produces electrical apparatus for industrial complexes....
-
What is the total entropy change accompanying a process in which 40.0 kJ of energy is transferred as heat from a large reservoir at 800. K to one at 200. K?
-
A good way to become familiar with thermodynamic processes is to start with a very simple system and consider how various changes might affect it. Calculate the change in molar Gibbs free energy, G m...
-
The composition of a compound used to make polyvinyl chloride (PVC) is 38.4% C, 4.82% H, and 56.8% Cl by mass. It took 7.73 min for a given volume of the compound to effuse through a porous plug, but...
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


