(a) Use a graphing calculator or standard graphing software to make an Arrhenius plot of the data...
Question:
(a) Use a graphing calculator or standard graphing software to make an Arrhenius plot of the data shown here for the decomposition of iodoethane into ethene and hydrogen iodide, C2H5I(g) → C2H4 (g) + HI(g), and determine the activation energy for the reaction.
(b) What is the value of the rate constant at 400°C?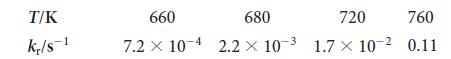
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





