(a) Use graphing software to plot standard potential against atomic number for the elements of Groups 1...
Question:
(a) Use graphing software to plot standard potential against atomic number for the elements of Groups 1 and 2.
(b) What generalizations can be deduced from the graph?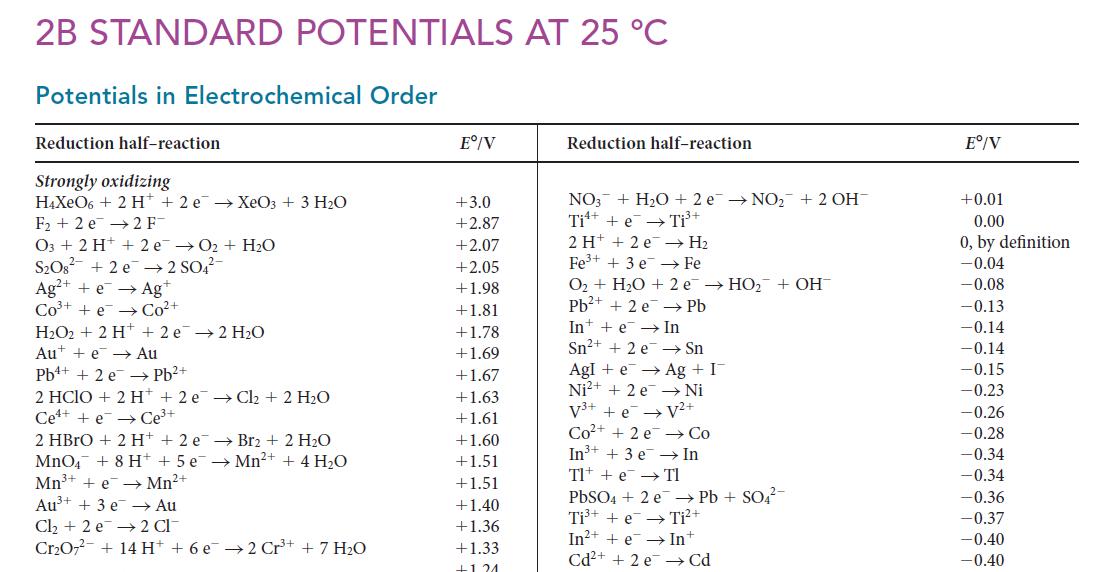
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 °C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6+ 2 H2e → XeO3 + 3 H₂O F₂2 e 2 F¯ O3 + 2 H + 2e →O₂+ H₂O S208² +2e →2 SO4²- Ag²+ + e- → Ag+ CO³+ +e Co²+ H₂O2 + 2 H+2e →2 H₂O Au + e→ Au Pb+ + 2e →→ Pb²+ 2 HCIO + 2 H+2 e→Cl₂ + 2 H₂O Ce+ e Ce³+ 2 HBrO+ 2 H+ +2 e → Br2 + 2 H₂O MnO4 + 8 H + 5 e → Mn²+ + 4 H₂O Mn³+ + e→→Mn²+ Au³+ + 3 e → Au Cl₂ + 2 e 2 Cl Cr₂O72 + 14 H+ + 6 e 2 Cr³+ + 7 H₂0 Eº/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +121 Reduction half-reaction NO3+ H₂O +2 e→NO₂+ 2 OH¯ Ti + e Ti³+ 2 H+ + 2e Fe³+ + 3 e →→ H₂ Fe O₂ + H₂O + 2 e → HO₂ + OH Pb²+ + 2 e Pb In + e →→ In Sn²+ + 2 e Sn Agle → Ag + I → Ni V²+ Ni²+ + 2e V³+ +e Co²+ +2 e In³+ + 3 e Tl + e Tl PbSO4 + 2 e →→ Pb + SO4²- Ti³+ + e Ti²+ In²+ + e→→ In+ Cd²+ + 2e →→ Cd Co In Eº/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a EV EV 25 26 27 28 29 30 31 16 18 20 22 24 26 28 30 I I T Li Be E Na K Rb ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use graphing software and data from Appendix 2B to plot ionization energy against standard potential for the elements of Groups 1 and 2. What generalizations can be drawn from the graph? 2B STANDARD...
-
To determine whether gene transfer from an organelle genome to the nucleus can be observed in the laboratory, a chloroplast transformation vector was constructed that contained two selectable...
-
The following accounts are showing for ABC Company. Cash = $16,080; Accounts receivable = $9,500; Accounts payable = $14,000; Supplies = $675; Prepaid expenses = $3,150; Equipment = $25,200;...
-
Question 3: Assume that Narine Inc. is considering leasing a car from Proctor Inc. for a period of four years. The fair value of the car today is $36,000. The current market interest rate for...
-
Table 6.8 shows investment and projected income in euros for Flanel's new perfume factory. Forecast cash flows and calculate NPV. The nominal cost of capital in euros is 11 percent. 2 3 6. 1. Capital...
-
Wish to design an FIR lowpass filter satisfying the specifications 0.95 < H(e j ) < 1.05, 0 || 0.25, 0.1 < H(e j ) < 0.1, 0.35 || , by applying a window w[n] to the impulse response h d [n] for...
-
Figure 20.34 shows a thin rod of length L carrying charge Q distributed uniformly over its length. (a) Whats the line charge density on the rod? (b) Modify the calculation of Example 20.7 to find an...
-
Top managers of City Video are alarmed by their operating losses. They are considering dropping the DVD product line. Company accountants have prepared the following analysis to help make this...
-
Please provide the journal entries and explanations! Using the accounting equation would be helpful to explain things. Thank you for your help, I will definitely leave a like! The truck in g was...
-
What are the sources for the production of helium and argon?
-
Is there any chemical support for the view that hydrogen should be classified as a member of Group 17? Give evidence that supports this view.
-
UCC Section 2-105 defines as all (tangible) things . . . which are movable at the time of identification to the contract for sale. I. Goods II. Fixtures III. Services IV. Property a. I b. II c. I and...
-
Name and define the more common constraints in any given project.
-
Graph the function f(x)=-x+4x-20 State where f(x) is increasing and decreasing. State any absolute extrema (if they exist). Determine the Domain and Range.
-
A residential wiring circuit is shown in the figure. In thismodel, the resistor R 3 is used to model a 250 V appliance(such as an electric range), and the resistors R 1 and R 2 are used to model 125...
-
1. The speed limit on some interstate highways is roughly 100 km/h. (a) What is this in meters per second? (b) How many miles per hour is this? 2. A car is traveling at a speed of 33 m/s. (a) What is...
-
Questions 33 and 34 are based on the following information: Bilog Company's budgeted fixed overhead costs are P50,000 and mthe variable factory overhead rate is P4 per direct labor hour. The standard...
-
Use the entire panel data set in AIRFARE.RAW for this exercise. The demand equation in a simultaneous equations unobserved effects model is Log(passenit) = it + 1 log(fareit) + ait + uit, where we...
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
Calculate the terms that can arise from the configuration np 1 np 1 , n n. Compare your results with those derived in the text for np 2 . Which configuration has more terms and why?
-
Derive the ground-state term symbols for the following configurations: a. d 5 b. f 3 c. p 4
-
As discussed in Chapter 20, in a more exact solution of the Schrödinger equation for the hydrogen atom, the coordinate system is placed at the center of mass of the atom rather than at...
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


