Use graphing software and data from Appendix 2B to plot ionization energy against standard potential for the
Question:
Use graphing software and data from Appendix 2B to plot ionization energy against standard potential for the elements of Groups 1 and 2. What generalizations can be drawn from the graph?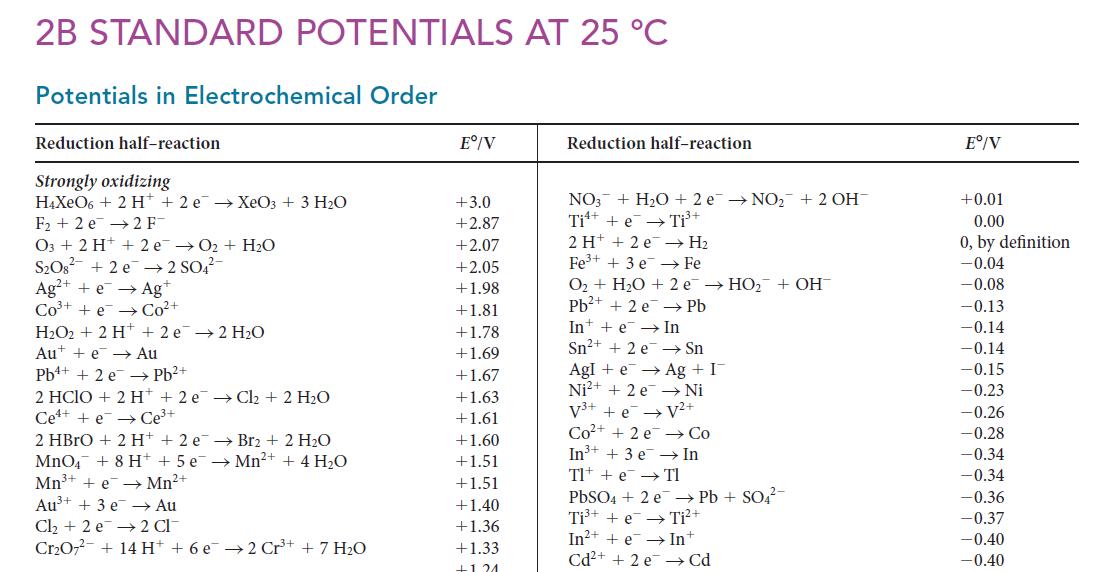
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+2e XeO3 + 3 HO F2 e 2 F 03 + 2 H+ 2e O + HO S208 +2e2 SO4- Ag+ + e Ag+ CO+ +e Co+ HO2 + 2 H+ + 2 e 2 HO Aue Au Pb+ + 2e Pb+ 2 HCIO + 2 H+2 eCl + 2 HO Ce++eCe+ 2 HBrO + 2 H+ + 2 e Br2 + 2 HO MnO4 + 8 H + 5 e Mn+ + 4 HO Mn+ + eMn+ Au+ + 3 e Au Cl +2 e 2 Cl CrO72 + 14 H +6e2 Cr+ + 7 HO E/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +121 Reduction half-reaction NO3 + HO +2 eNO+ 2 OH Ti + e Ti+ 2 H + 2e Fe+ + 3 e O + HO + 2 e HO + OH Pb+ + 2 e Pb In + e In Sn+ + 2e H Fe Sn Agle Ag + I Ni V+ Ni+ + 2e V+ +e Co+ +2 e In+ + 3 e Tl + e PbSO4 + 2e Ti+ + e In+ + e Cd+ +2e Co In Tl Pb + SO4- Ti+ Int Cd E/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
I kJmol 950 850 750 650 Ca 550 Li Bal 450 Mg Be Rb Cs 350 32 3 28 2...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
(a) Use graphing software to plot standard potential against atomic number for the elements of Groups 1 and 2. (b) What generalizations can be deduced from the graph? 2B STANDARD POTENTIALS AT 25 C...
-
K sp for Ni(OH) 2 is 6.5 * 10 18 . Use this value and data from Appendix 2B to calculate E for the half-reaction Ni(OH) 2 (s) + 2 e Ni(s) + 2 OH (aq). 2B STANDARD POTENTIALS AT 25 C Potentials in...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
please solve it in details with clear explanation for each step John made a deposit of 4400 into a fund at the beginning of each year for 16 years. At the end of 16 years, he began making semiannual...
-
In 1898 Simon North announced plans to construct a funeral home on land he owned and rented out as a storage area for railway carts. (A local newspaper commended Mr. North for not putting the cart...
-
Consider a stable linear time-invariant system with a real input x[n], a real impulse response h[n], and output y[n]. Assume that the input x[n] is white noise with zero mean and variance 2 x . The...
-
Differentiate Equation 29.12 with respect to x and Equation 29.13 with respect to t. Then, using the fact that mixed derivatives are equal (e.g., ), combine the resulting equations and show that the...
-
Two different analytical tests can be used to determine the impurity level in steel alloys. Eight specimens are tested using both procedures, and the results are shown in the following tabulation. Is...
-
Required information [The following information applies to the questions displayed below.] Three years ago, Adrian purchased 310 shares of stock in X Corp. for $35,340. On December 30 of year 4,...
-
Identify the oxidation number of the halogen atoms in (a) Iodine heptafluoride; (b)Sodium periodate; (c)Hypobromous acid; (d) Sodium chlorite.
-
State which element of each of the following pairs is more electronegative: (a) Sulfur, phosphorus; (b) Selenium, tellurium; (c) Sodium, cesium; (d) Silicon, oxygen.
-
Referring to the situation in Question 2, one might think that an informative prior would outweigh the effect of the increasing sample size.With respect to the Bayesian analysis of the linear...
-
I Need Hr project on Employee Engagement What is Employee Engagement and how does it contribute to organizational success? What is the role of HR in improving employee engagement? What are some...
-
In the United States, the Veterans Administration (VA) is tasked with, among other things, providing quality health care for U.S. military veterans. Chronically underfunded, the agency was having...
-
Using the keywords you brainstormed earlier in the module, conduct three separate searches in CQ Researcher - SAGE, Academic Search Ultimate, or another relevant database. When conducting these...
-
TechEx Repair allows local hardware stores to expand their service offerings to their customers by providing an off-site small engine repair service. Customers bring in small engines such as lawn...
-
Purpose: Sometimes we are asked to collaborate with a team of writers. This collaboration can help us to understand how others think differently from us and help us to think more creatively. This...
-
Use the data in PHILLIPS.RAW for this exercise. (i) In Example 11.5, we estimated an expectations augmented Phillips curve of the form inft = 0 + 1 unemt + et, where inft = inft - inft-1. In...
-
Explain how two samples can have the same mean but different standard deviations. Draw a bar graph that shows the two samples, their means an standard deviations as error bars. T S
-
Without invoking equations, explain why the energy of the triplet state is lower than that of the singlet state for He in the 1s 1 2s 1 configuration.
-
Justify the statement that the Coulomb integral J defined in Equation (22.20) is positive by explicitly formulating the integral that describes the interaction between two negative classical charge...
-
Explain why the first ionization energy and electron affinity for F are larger than for O.
-
Marie Forleo, a marketing trainer and host of MarieTV, presents the eight tips for genuine networking. Do you agree or disagree with her suggestions? Discuss how this information is useful to you and...
-
Identify all relevant costs or revenue that are applicable to production- constrained decisions 1. Contributions margin of product 2. Interference with other production 3. Contribution margin per...
-
Gammaro Compary manufactures wallets from fabric. In 2 0 1 9 , Gammaro made 2 , 1 5 0 , 0 0 0 wallets using 1 , 2 5 0 , 0 0 0 yards of fabric. In 2 0 1 9 , Gammaro has capacity to make 2 , 8 0 0 , 0...

Study smarter with the SolutionInn App


