An aqueous solution of Na 2 SO 4 was electrolyzed for 30.0 min; 25.0 mL of oxygen
Question:
An aqueous solution of Na2SO4 was electrolyzed for 30.0 min; 25.0 mL of oxygen was collected at the anode over water at 22°C and a total pressure of 722 Torr. Determine the current that was used to produce the gas. See Table 5A.2 for the vapor pressure of water.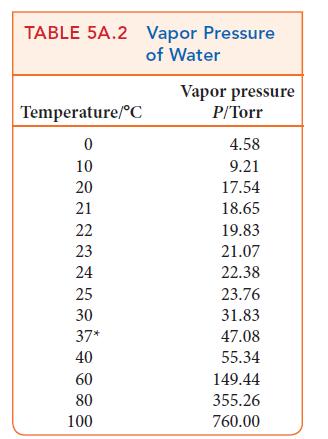
Transcribed Image Text:
TABLE 5A.2 Vapor Pressure of Water Temperature/C 0 10 20 21 22 23 24 25 30 37* 40 60 80 100 Vapor pressure P/Torr 4.58 9.21 17.54 18.65 19.83 21.07 22.38 23.76 31.83 47.08 55.34 149.44 355.26 760.00
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
On January 1, 2022, the ledger of Sandhill Co. contained these liability accounts. Accounts Payable $43,500 Sales Taxes Payable 7,600 Unearned Service Revenue 20,000 During January, the following...
-
Various uses for nitric acid are given in Problem 6.43, along with information about how this important chemical is synthesized industrially. The key reactions are oxidations of ammonia to nitric...
-
An aqueous solution of ammonium nitrite, NH4NO2, decomposes when heated to give off nitrogen, N2. NH4NO2(s) 2H2O(g) + N2(g) This reaction may be used to prepare pure nitrogen. How many grams of...
-
Show that 15 is an inverse of 7 modulo 26.
-
Division managers compensation, levers of control (continuation of 23-32). Murdoch Turner seeks your advice on revising the existing bonus plan for division managers of News Mogul Group. Assume...
-
A spherical ball of charged particles has a uniform charge density. In terms of the ball's radius R, at what radial distances (a) Inside and (b) Outside the ball is the magnitude of the ball's...
-
How many years of experience in this business and how many years of total experience do they have?
-
Using Table 6-13, what is required new financing if next years sales forecast increases to $400,000, profit margin is 10 percent, and the payout ratio is 90 percent? Table 6-13 Total Sales Forecast...
-
0 Problem 8 - 2 6 Calculating NPV and IRR for a Replacement A firm is considering an investment in a new machine with a price of $ 1 8 . 1 5 million to replace its existing machine. The current...
-
A manager of a store that sells and installs spas wants to prepare a forecast for January, February, and March of next year. Her forecasts are a combination of trend and seasonality. She uses the...
-
The magnitudes of the standard potentials of two metals M and X were determined to be When the two electrodes are connected, current flows from M to X in the external circuit. When the electrode...
-
Consider the electroplating of a metal +1 cation from a solution of unknown concentration according to the half-reaction M + (aq) + e M(s), with a standard potential E. When the half-cell is...
-
In determining whether control procedures are potentially reliable in assessing control risk below the maximum, an auditor performs four tasks, listed below in random order: 1. Design tests of...
-
Molina Company produces three products: A130, B324, and C587 All three products use the same direct material, Brac Unt data for the three products are in the provided table. (Click to view the unit...
-
MFGE 437 S21 - Homework 1 Submissions will be Online! Please scan your HWs and upload on Canvas Problem 1: A vertical milling machine is to be retrofitted with three identical DC servo motors. The...
-
On January 1, Palisades, Inc., acquired 100 percent of Sherwood Company's common stock for a fair value of $120,340,000 in cash and stock. The carrying amounts of Sherwood's assets and liabilities...
-
(1) A test balloon has an accelerometer attached to it. After you release it and start collecting data it is 5 ft in front of you and 16 ft above you, and it is moving 5 ft/s to your left and 4 ft/s...
-
484 ... Age of Accounts as of June 30, 2019 1-30 31-60 61-90 Over 90 Customer Name Days Days Days Days Total Balance Canyon Youth Club $ 250 $ 250 Crazy Tees 200 $ 150 350 Early Start Daycare $500...
-
For each of the following situations, suppose H0: 1 2 is being tested against HA: 1 2.-State whether or not there is significant evidence for HA. (a) P-value = 0.046, = 0.02. (b) P-value = 0.033, ...
-
In the operation of an automated production line with storage buffers, what does it mean if a buffer is nearly always empty or nearly always full?
-
A camper stranded in snowy weather loses heat by wind convection. The camper is packing emergency rations consisting of 58% sucrose, 31% fat, and 11% protein by weight. Using the data provided in...
-
Calculate H o R and U o R at 298.15 K for the following reactions: a. 4NH 3 (g) + 6NO(g) 5N 2 (g) + 6H 2 O(g) b. 2NO(g) + O 2 (g) 2NO 2 (g) c. TiCl 4 (l) + 2H 2 O(l) TiO 2 (s) + 4HCl(g) d....
-
At 1000.K, H o R = 123.77 kJ mol 1 for the reaction N 2 (g) + 3H 2 (g) 2NH 3 (g), with C P,m = 3.502R, 3.466 R, and 4.217 R for N 2 (g), H 2 (g), and NH 3 (g), respectively. Calculate H o f of NH 3...
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


