Ant venom contains formic acid (HCOOH; formica is the Latin word for ant). Suppose you are at
Question:
Ant venom contains formic acid (HCOOH; “formica” is the Latin word for ant). Suppose you are at a pharmaceutical company working on a quick antidote and need to estimate the pH at the stoichiometric point when titrating a solution of formic acid. Estimate the pH at the stoichiometric point of the titration of 25.00 mL of 0.100 m HCOOH(aq) with 0.150 m NaOH(aq).
ANTICIPATE The formate ion produced in this titration is a base, so you should expect pH > 7.
PLAN To calculate the pH at the stoichiometric point, proceed as in Examples 6D.4 or 6D.5. Note that the amount of salt at the stoichiometric point is equal to the amount of titrant added and that the volume is the total volume of the combined analyte and titrant solutions. The Kb of a weak base is related to the Ka of its conjugate acid by Ka * Kb = Kw; the Ka is listed in Table 6C.1.
What should you assume? Assume that the autoprotolysis of water has no significant effect on the pH and that because the formate ion is a very weak base, its concentration changes insignificantly when it is protonated by water.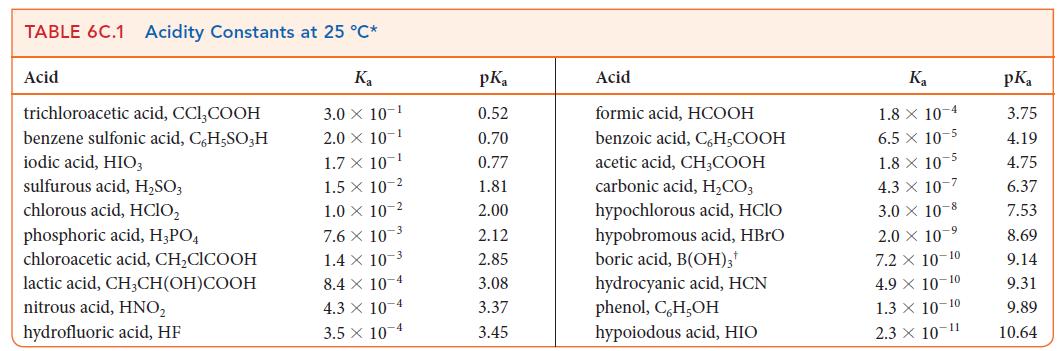
Examples 6D.4
You are working in the emergency room of a hospital where a patient suffering from influenza has developed metabolic alkalosis, a condition in which the pH of the blood is too high. You have available a stock solution of ammonium chloride, which is used to lower the pH of the blood of patients suffering from alkalosis, but you need to know its pH. Estimate the pH of 0.15 m NH4Cl(aq) at 25°C.
ANTICIPATE Because NH4+ is a weak acid and Cl– is neutral, you should expect pH
PLAN Treat the solution as that of a weak acid, using an equilibrium table as in Toolbox 6D.1 to calculate the composition and hence the pH. First, write the chemical equation for proton transfer to water and the expression for Ka. Obtain the value of Ka from Kb for the conjugate base by using Eq. 4a in Topic 6C (Ka = Kw/Kb). The initial concentration of the acidic cation is equal to the concentration of the cation that the salt would produce if it retained all its acidic protons.
What should you assume? Assume that (1) the extent of deprotonation is so small that the change in concentration of NH4+ is insignificant, and (2) the autoprotolysis of water does not affect the pH significantly. Check these assumptions at the end of the calculation.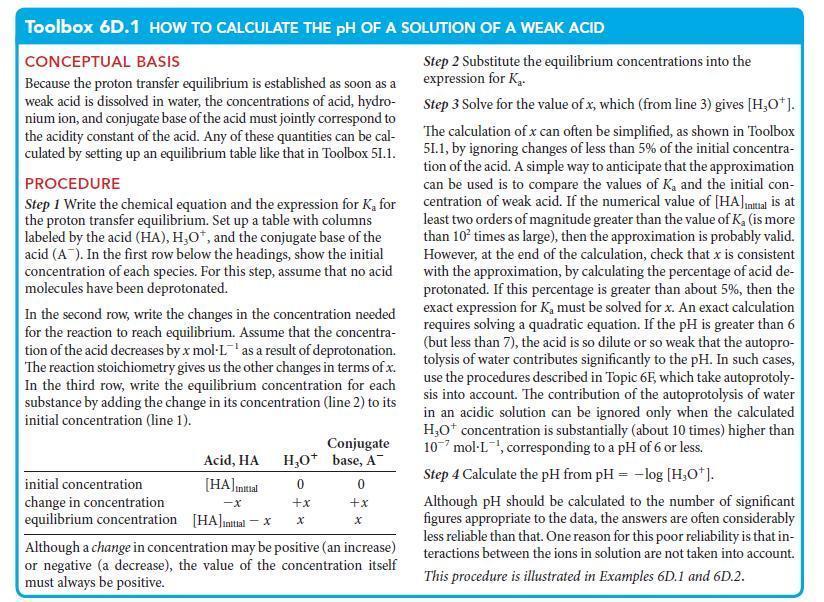
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





