At what temperatures is the following process spontaneous at 1 atm? What is the normal boiling point
Question:
At what temperatures is the following process spontaneous at 1 atm?
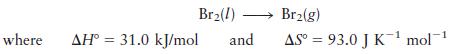
What is the normal boiling point of liquid Br2?
Transcribed Image Text:
Br(1) Br(g) where AH 31.0 kJ/mol and AS= 93.0 J K mol-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The vaporization process will be spontaneous at all temperatures at which AG is negative Note that A...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Sunshine Service Center received a 120-day, 6% note for $40,000 dated April 12 from a customer on account. Assume a 360 day year. a) Determine the due date of the note?? b) Determine the maturity...
-
Like most substances, bromine exists in one of the three typical phases. Br2 has a normal melting point of 27.2oC and a normal boiling point of 59oC. The triple point for Br2 is 27.3oC and 40 torr,...
-
Liquid nitrogen is stored in 0.5-m3 metal tanks that are thoroughly insulated. Consider the process of filling an evacuated tank, initially at 295 K. It is attached to a line containing liquid...
-
In the United States, a principal responsibility for preserving endangered species (e.g., a pair of endangered birds that chooses to nest on private land) and the costs of exercising that...
-
A clown is juggling four balls simultaneously. Students use a video tape to determine that it takes the clown 0.9 s to cycle each ball through his hands (including catching, transferring, and...
-
Interpret the phrase "that which gets watched, gets done." Give an example from your personal life?
-
Have you used a social network or similar site to interact with, or make comments about, an organisation?
-
Mr. Gennone maintained a checking account at Peoples National Bank & Trust Company of Pennsylvania (Bank). Gennone noticed that he was not receiving his bank statements and canceled checks. When...
-
Top executive officers of Adams Company, a merchandising firm, are preparing the next years budget. The controller has provided everyone with the current years projected income statement. Current...
-
Predict the sign of S for each of the following reactions. a. the thermal decomposition of solid calcium carbonate: b. the oxidation of SO 2 in air: CaCO3(s) CaO(s) + CO,(g)
-
In the metallurgy of antimony, the pure metal is recovered by different reactions, depending on the composition of the ore. For example, iron is used to reduce antimony in sulfide ores: Carbon is...
-
Why do you think organizations that have a comprehensive mission tend to be high performers? Does having a comprehensive mission cause high performance?
-
Charlotte, a marketing manager, is worried her firm is doing a poor job of managing the movement of finished products to the final consumer. If she is right, the company should work to improve its Mul
-
Sketch a graph of the piecewise defined function. f(x) = Sx if x 0 x+9 if x>0
-
Complete the information for the following subnetting problem. Your answers should be whole numbers without a period. Example: the correct value for the last octet of 172.20.55.210 would be entered...
-
Olympia Trophy Company wants to purchase a laser engraving machine to use in its production of trophies. The cost of this engraving machine is $69,546.40 and it will yield yearly expected cash flows...
-
Scenario: Envirotruck is a company that produces energy efficient all-wheel-drive and 4-wheel-drive trucks (twice as efficient as their competition's) with ample clearance for construction and rough...
-
Why is the Carnot cycle not a realistic model for steam power plants?
-
Outline a general process applicable to most control situations. Using this, explain how you would develop a system to control home delivery staff at a local pizza shop.
-
Identify the number of valence electrons (including d-electrons) present in each of the following metal ions: (a) Co 2+ ; (b) Mo 4+ ; (c) Ru 4+ ; (d) Pt 2+ ; (e) Os 3+ ; (f) V 3+ .
-
The complex ion [Ni(NH 3 ) 6 ] 2+ forms in a solution containing 0.16 mol L 1 NH 3 (aq) and 0.015 mol L 1 Ni 1 (aq). If the formation constant of [Ni(NH 3 ) 6 ] 2+ is 1.0 * 10 9 , what are the...
-
Name each of the following complex ions and identify the oxidation number of the metal: (a) [CrCl 3 (NH 3 ) 2 (OH 2 )] + ; (b) [Rh(en) 3 ] 3+ ; (c) [Fe(Br) 4 (ox)] 3 ; (d) [Ni(OH)(OH 2 ) 5 ] 2+ .
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
Stock in ABC has a beta of 0.9. The market risk premium is 8%, and T-bills are currently yielding 5%. The company's most recent dividend is $1.60 per share, and dividends are expected to grow at a 6%...
-
Sweeten Company had no jobs in progress at the beginning of March and no beginning inventories. The company has two manufacturing departments --Molding and Fabrication. It started, completed, and...

Study smarter with the SolutionInn App


