Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take
Question:
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in basic solution. Identify the oxidizing agent and reducing agent in each reaction.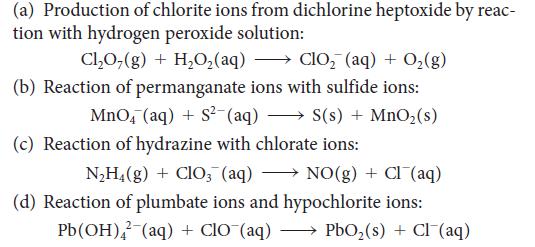
Transcribed Image Text:
(a) Production of chlorite ions from dichlorine heptoxide by reac- tion with hydrogen peroxide solution: Cl₂O(g) + H₂O₂(aq) → ClO₂ (aq) + O₂(g) (b) Reaction of permanganate ions with sulfide ions: MnO4 (aq) + S²-(aq) →→→ S(s) + MnO₂(s) (c) Reaction of hydrazine with chlorate ions: N₂H4(g) + ClO3(aq) NO(g) + Cl(aq) (d) Reaction of plumbate ions and hypochlorite ions: Pb(OH)2 (aq) + CIO¯(aq) →→→→ → PbO₂ (s) + Cl¯(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Here are the balanced equations with the oxidizing agent in boldface and the reducing agent i...View the full answer

Answered By

Rajat Gupta
used to take tution classes from my school time.
Conducted special topic claases during my graduation to help the students pass their exams.
Currently, teaching and conducting online claases during my post- graduation too.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in basic solution. Identify the oxidizing agent and reducing agent in...
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in...
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in...
-
The CFO of the Jordan Microscope Corporation intentionally misclassified a downstream transportation expense in the amount of $575,000 as a product cost in an accounting period when the company made...
-
Weighted-average method. Porter Handcraft is a manufacturer of picture frames for large retailers. Every picture frame passes through two departments: the Assembly Department and the Finishing...
-
Consider the binary symmetric channel described in Figure. Let Po denote the prob. ability of sending binary symbol x 0 = 0, and let p 1 = 1 ? P 0 denote the probability of sending binary symbol x 1...
-
Calculate the correlation coefficient r.
-
Coastal Computer operates two retail outlets in Oakview, one on Main Street and the other in Lakeland Mall. The stores share the use of a central accounting department. The cost of the accounting...
-
The Adjusted Trial Balance section of the worksheet for Van Zant Janitorial Supplies follows. The owner made no additional \ table [ [ VAN ZANT JANITORIAL SUPPLIES ] , [ Post - closing Trial Balance...
-
1- The outflow concentration from a reactor is measured at a number of times over a 24-hr period: t, hr 5.5 10 12 14 16 18 20 24 C, mg/ 1.5 2.3 2.1 4 5 5.5 5 3 1.2 The flow rate for the outflow in...
-
Suppose that there are typically 20 average-sized drops in 1.0 mL of an aqueous solution. Will a precipitate form when 1 drop of 0.010 m NaCl(aq) is added to 10.0 mL of (a) 0.0040 m AgNO 3 (aq); (b)...
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A total charge of 67.2...
-
Which of the following is used to create an instance of an array using reflection? (a) Array.newInstance() (b) Array.instance() (c) Array.createArray() (d) Array.createInstance()
-
3) A spider crawls with constant speed vo on a phonograph turntable rotating with constant angular speed w in the xy plane on a radially outward path, relative to the centre of the turntable. The...
-
Question Encik Zubir ( a certified handicapped person ) is the owner of a financial consulting firm, Bijak Wealth Enterprise. The business assists its clients to grow their wealth. Encik Zubir is...
-
What is XYZ Corp.'s net cash flow XYZ Corp. (for 2020) Revenue $5,000,000 Wages: $1,000,000 D&A: $1,000,000 Property, Plant & Equipment investment: $1,500,000 Tax Rate: 35% NOWC (2020): $750,000 NOWC...
-
Tower x (m) y (m) UU3 -118.1 -15.6 OU1 -85.3 -15.9 Sensor heights (m) 3.19, 4.16, 5.04, 7.24, 9.84 1.5, 3.0, 5.46, 9.86, 15.65 OU2 -90.0 -8.3 1.5, 2.96, 5.97, 9.91, 15.08 ASU -22.8 -8.6 5.0 UUT -13.3...
-
For each of the matrices determine the value(s) of c for which the given matrix is not invertible. [4 25. 26. 3 5 } ] 6 27. 28. 2 c+4 C -8 c-6]
-
Calculate the SD of each of the following fictitious samples: (a) 11, 8, 4, 10, 7 (b) 23, 29, 24, 21, 23 (c) 6, 0, - 3, 2, 5
-
The domain of the variable in the expression x 3/x + 4 is________.
-
According to the 3rd postulate, in any single measurement of the total energy, the only values that will ever be measured are the eigenvalues of the total energy operator. Apart from the discrete...
-
Why must an acceptable wave function be single valued?
-
Why must the first derivative of an acceptable wave function be continuous?
-
Glencove Company makes one model of radar gun used by law enforcement officers. All direct materials are added at the beginning of the manufacturing process. Information for the month of September...
-
Larren Buffett is concerned after receiving her weekly paycheck. She believes that her deductions for Social Security, Medicare, and Federal Income Tax withholding (FIT) may be incorrect. Larren is...
-
The major justification for adding Step 0 to the U.S. GAAP impairment test for goodwill and indefinite lived intangibles is that it: A. Saves money spent estimating fair values B. Results in more...

Study smarter with the SolutionInn App


