Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take
Question:
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in basic solution. Identify the oxidizing agent and reducing agent in each reaction.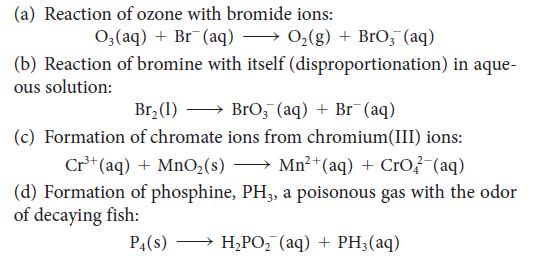
Transcribed Image Text:
(a) Reaction of ozone with bromide ions: O₂ (aq) + Br (aq) → O₂(g) + BrO₂ (aq) (disproportionation) in aque- (b) Reaction of bromine with itself ous solution: Br₂ (1) →BrO₂ (aq)+ Br(aq) (c) Formation of chromate ions from chromium(III) ions: Cr³+ (aq) + MnO₂ (s) Mn²+ (aq) + CrO2 (aq) (d) Formation of phosphine, PH3, a poisonous gas with the odor of decaying fish: P4(S) H₂PO₂ (aq) + PH3 (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a 3 03g Braq 3 0g BrO aq is the oxidizing agent and Br is the reducing ...View the full answer

Answered By

CHARLES AMBILA
I am an experienced tutor with more than 7 years of experience. I have helped thousands of students pursue their academic goals. My primary objective as a tutor is to ensure that students have easy time handling their academic tasks.
5.00+
109+ Reviews
324+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in basic solution. Identify the oxidizing agent and reducing agent in...
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in...
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in...
-
Solve this system of equations -3x-y 5 -3x - 4y 83 || y = 1 11
-
Market-share and market-size variances (continuation of 14-25) Soda-King prepared the budget for 2009 assuming a 10% market share based on total sales in the western region of the United States. The...
-
Construct a Venn diagram to illustrate the possible intersections and unions for the following events relative to the sample space consisting of all automobiles made in the United States. F: Four...
-
Explain why you agree or disagree with some of the suggestions in this chapter for improving project communications, such as creating a communications management plan, stakeholder analysis, or...
-
Goodwin Auto Supply does not segregate sales and sales taxes at the time of sale. The register total for March 16 is $13,440. All sales are subject to a 5% sales tax. Compute sales taxes payable, and...
-
On January 1, 2024, White Water issues $600,000 of 7% bonds, due in 10 years, with interest payable annually on December 31 each year. Assuming the market interest rate on the issue date is 6%, the...
-
Warf Computers, Inc., was founded 15 years ago by Nick Warf, a computer programmer. The small initial investment to start the company was made by Nick and his friends. Over the years, this same group...
-
Calculate the pH of 8.23 * 10 7 m NaNH 2 (aq)
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A total charge of 4.5 kC...
-
Would you welcome happiness training in your workplace? Why or why not?
-
1. Make a comparison between the leadership approaches "Trait Models" and "Behavioral Models". Discuss the main postulates and differences between the models and provide examples of a theory...
-
Applying the Central Limit Theorem: The amount of contaminants that are allowed in food products is determined by the FDA ( Food and Drug Administration ) . Common contaminants in cow milk include...
-
A collection of techniques used by social scientists to compile, summarize, and convey numerical data. Revised Research Question Hypothesis : Null Hypothesis : A null hypothesis, often known as H0,...
-
Adidas is an international sporting apparel/shoes brand. If Adidas was to enter a new foreign market, it would conduct a country market assessment. Identify the 4 components of the assessment....
-
Using the scenario linked in the Supporting Materials section, assume that you are the cost accountant for your company, and the CFO has asked for your analysis on purchasing materials from an online...
-
Refer to Example 5.3.3. In the sampling distribution of for n = 4 (Figure 5.3.4), approximately what is the area under (a) The first peak? (b) The second peak?
-
A fuel pump sends gasoline from a car's fuel tank to the engine at a rate of 5.88 10-2 kg/s. The density of the gasoline is 735 kg/m3, and the radius of the fuel line is 3.18 10-3 m. What is the...
-
In the DebyeHckel theory, the counter charge in a spherical shell of radius r and thickness dr around the central ion of charge +Q is given by Q 2 re r dr. Calculate the radius at which the counter...
-
Calculate the solubility of CaCO 3 (K sp = 3.4 10 -9 ) a. In pure H 2 O. b. In an aqueous solution with I = 0.0250 mol kg 1 . For part (a), do an iterative calculation of and the solubility until...
-
Calculate the probability of finding an ion at a distance greater than 1/ from the central ion.
-
American Food Services, Incorporated leased a packaging machine from Barton and Barton Corporation. Barton and Barton completed construction of the machine on January 1 , 2 0 2 4 . The lease...
-
Which of the following statements is true? Financial measures tend to be lag indicators that report on the results of past actions. LA profit center is responsible for generating revenue, but it is...
-
Andretti Company has a single product called a Dak. The company normally produces and sells 8 0 , 0 0 0 Daks each year at a selling price of $ 5 6 per unit. The company s unit costs at this level of...

Study smarter with the SolutionInn App


