Barium metal is produced by the reaction of aluminum metal with barium oxide. From the standard reaction
Question:
Barium metal is produced by the reaction of aluminum metal with barium oxide. From the standard reaction enthalpies,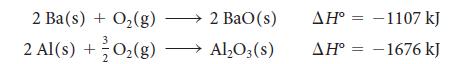
calculate the reaction enthalpy for the production of metallic barium in the reaction![]()
Transcribed Image Text:
2 Ba(s) + O₂(g) 2 Al(s) +O₂(g) 2 BaO(s) Al₂O3(s) AH° -1107 kJ AH° -1676 kJ = = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Aluminum metal reacts with iron(III) oxide to produce aluminum oxide and iron metal. a. How many moles of Fe2O3 are required to completely react with 41 g Al? b. How many moles of Fe are produced by...
-
Aluminum metal reacts with iron(III) oxide to produce aluminum oxide and iron metal. a. How many moles of Fe 2 O 3 are required to completely react with 44 g Al? b. How many moles of Fe are produced...
-
Monochlorobenzene is produced by the reaction of benzene with chlorine. A mixture of monochlorobenzene and dichlorobenzene is produced, with a small amount of trichlorobenzene. Hydrogen chloride is...
-
On January 1, 2012, Wilmes Floral supplies borrowed $2,413 from Bower Financial Services. Wilmes Floral Supplies gave Bower a $2,500 note with a maturity date of December 31, 2013. The note specified...
-
(a) Eve Adams believes the production cost report is an external report for stockholders. Is Eve correct? Explain. (b) Identify the sections in a production cost report.
-
Evaluate the integral. dt tV1? - 16
-
E 16-20 Recording new partner investment After operating as partners for several years, Gro and Ham decided to sell one-half of each of their partnership interests to Lot for a total of $70,000, paid...
-
What items should be included in the audit status report?
-
What effect does a professional body member's improper behavior have on the member, the professional body, and the profession
-
An ice cube of mass 50.0 g at 0.0 C is added to a glass containing 400.0 g of water at 45.0C. What is the final temperature of the system? Assume that no heat is lost to the surroundings.
-
Determine the reaction enthalpy for the hydrogenation of ethyne to ethane, C 2 H 2 (g) + 2 H 2 (g) C 2 H 6 (g), from the following data: H c (C 2 H 2 , g) = 1300. kJ mol 1 , H c (C 2 H 6 , g) =...
-
Perform the indicated operation, and write each answer in lowest terms.
-
Which alternative strategy do each of the following fall under? 1. Nike could set more aggressive sustainability targets and timelines for each product category, allocating additional resources to...
-
Find the critical value Za/2 that corresponds to the given confidence level. 88%
-
A study was conducted to determine the proportion of people who dream in black and white instead of color. Among 296 people over the age of 55, 73 dream in black and white, and among 294 people under...
-
The other strategy could be to develop a completely distinct product line. This would allow Nike to develop sustainable products without affecting their main products. It could target specific green...
-
A drug is used to help prevent blood clots in certain patients. In clinical trials, among 4705 patients treated with the drug, 170 developed the adverse reaction of nausea. Construct a 95% confidence...
-
Genes that cause Prader-Willi syndrome and Angelman syndrome are closely linked along chromosome 15. Although people with these syndromes do not usually reproduce, let's suppose that a couple...
-
We all experience emotions, but some people disguise their true feelings better than others. Do you think this is a helpful or harmful thing to do? Under what conditions do you think it would be most...
-
Is ocranoic acid more soluble in 1 M HC1, 1 M NaOH, or pure water? Explain. Drugs such as morphine are often treated with strong acids. The most commonly used form of morphine is morphine...
-
Consider the reaction to produce the ester methyl acetate: When this reaction is carried our with CH3OH contain¬ing radioactive oxygen-18, the water produced does not contain oxygen-18. Explain...
-
A compound containing only carbon and hydrogen is 85.63% C by mass. Reaction of this compound with H2O produces a secondary alcohol as the major product and a primary alcohol as the minor product. If...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


