Below are molecular models of two oxoacids. Write the name of each acid and then draw the
Question:
Below are molecular models of two oxoacids. Write the name of each acid and then draw the model of its conjugate base.
(Red = O, white = H, green = Cl, and blue = N.)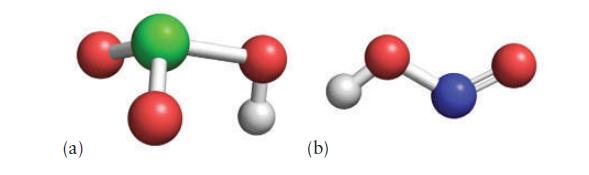
Transcribed Image Text:
(a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a HClO3 chloric ...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the molar mass of the following substances. a. H ON c. (NH4)2Cr2O7 b. HZ N
-
A seasonal food processing industry currently meets its demand for heat and electrical power by purchasing electricity from the local utility and generating heat from a natural gas fired boiler. The...
-
Below are molecular models of two oxoacids. Write the name of each acid and then draw the model of its conjugate base. (Red = O, white = H, green = Cl, and blue = N.) (a) (b)
-
The owner of Atlantic City Confectionary is considering the purchase of a new semiautomatic candy machine. The machine will cost $25,000 and last 10 years. The machine is expected to have no salvage...
-
Supply chain effects on total relevant inventory cost. Cow Spot Computer Co. outsources the production of motherboards for its computers. It has narrowed down its choice of suppliers to two...
-
Consider X 1 , X 2 , ., X n independent Poisson random variables with parameters 1 , 2 , , n. Use the properties of moment-generating functions to show n that the random variable n i = 1 Xi is...
-
Describe the criterion for rejecting the null hypothesis when using the p-value method for hypothesis testing. Who chooses the value of the level of significance, ????? Make up a situation (one...
-
Some fast-food chains offer a lower-priced combination meal in an effort to attract budget-conscious customers. One chain test-marketed a burger fries and a drink combination for $1.71. The weekly...
-
Required information [The following information applies to the questions displayed below.] Income statement and balance sheet data for Great Adventures, Inc., are provided below. GREAT ADVENTURES,...
-
1. Why wasnt money an adequate remedy in this case? 2. What does Wilcox mean when he alleges that Shollmier engaged in collusion? 3. How could Wilcox have prevented the property from being sold below...
-
Arrange the following metals in order of increasing strength as reducing agents for species in aqueous solution: (a) Cu, Zn, Cr, Fe; (b) Li, Na, K, Mg; (c) U, V, Ti, Al; (d) Ni, Sn, Au, Ag.
-
The pH of several solutions was measured in the research laboratories of a food company; convert each of the following pH values into the molar concentration of H 3 O + ions: (a) 3.3 (the pH of sour...
-
Wood Again Inc. produces tables from recycled wood in a three-stage process that includes cutting, assembling, and finishing, in that order. Direct materials are added in the Cutting and Finishing...
-
Solve the following linear system by Gaussian elimination with back-substitution without introducing fractions in your row-reduction. If there is no solution, explain why. -3x+8y + 82 = -8 -2x+ y -...
-
Introduction Some predictions are a slam dunk. Retail will continue to be driven by technology. Science fiction is coming to life in the form of robotics and virtual reality. And the Internet will...
-
Oswego Clay Pipe Company provides services of $ 5 0 , 0 0 0 to Southeast Water District # 4 5 on April 1 2 of the current year with terms 1 / 1 5 , n / 6 0 . What would Oswego record on April 1 2 ?...
-
Assume the following excerpts from a company's balance sheet: Property, plant, and equipment Beginning Balance $3,500,000 Ending Balance $3,700,000 $1,100,000 $800,000 Long-term investments During...
-
On January 1, 2021, Bonita Corp. had472,000shares of common stock outstanding. During 2021, it had the following transactions that affected the Common Stock account. February 1 Issued 125,000shares...
-
The following data table is taken from the study reported in Exercise 3.3.1. Here "stressed" means that the pei son reported that most days are extremely stressful or quite stressful; "not stressed"...
-
What are some of the various ways to implement an awareness program?
-
When aniline is treated with a mixture of nitric acid and sulfuric acid, the expected nitration product (para-nitroaniline) is obtained in poor yield. Instead, the major product from nitration is...
-
Starting with nitrobenzene and using any other reagents of your choice, outline a synthesis of para-chloroaniline.
-
Using acetic acid as your only source of carbon atoms, show how you could make N-ethyl acetamide. ,
-
Production numbers for 2 shifts are shown. The shift supervisor of Shift 2 insists to the production manager that her operators are more productive than the ones on Shift 1. Using a confidence level...
-
In a class, the scores that students got are as shown. What are the 25, 50, 75 and 100th percentiles for the data? 84 84 98 80 89 83 85 56 85 84 84 74 84 81 83 80 45 86 67 79 81 78 76 85 83 77 86 83...
-
Number of points made by Teams A and B are shown. Which statement is true based on running the F-Test Two-Sample for Variances in the Data Analysis pack in Excel? Use a confidence level of 10% to...

Study smarter with the SolutionInn App


