By assuming that the lattice enthalpy of NaCl 2 is the same as that of MgCl 2
Question:
By assuming that the lattice enthalpy of NaCl2 is the same as that of MgCl2, use enthalpy arguments based on data in Appendix 2A, explain why NaCl2 is an unlikely compound.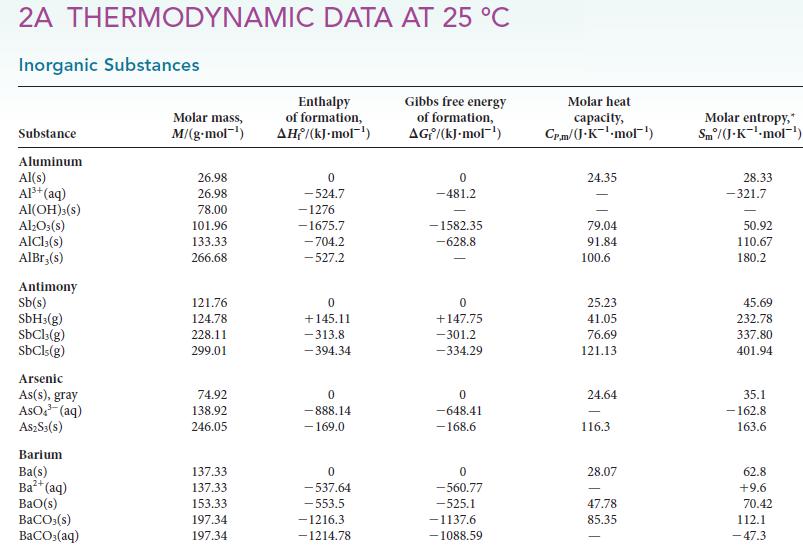
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) Al(OH)3(S) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO4³ (aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy,* Sm/(J-K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
In order to assess the likelihood of the existence of NaCl by assuming that its lattice enthalpy is ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Based on data in Table 8.2, estimate (within 30 kJ / mol) the lattice energy for (a) LiBr, (b) CsBr, (c) CaCl2. TABLE 8.2 Lattice Energies for Some Ionic Compounds Lattice Energy (kJ/mol) Lattice...
-
In Problem 15-48, you were asked to use best subsets stepwise regression to establish the relationship between body fat and the independent variables weight, abdomen circumference, and thigh...
-
The lattice enthalpy of sodium chloride, H for NaCl(s) Na+(g) + Cl(g)
-
ABC Company produces and sells I product. Once the products are produced, they are sold, and there is no work-in- process, no any inventory in stock. Company uses standard costing method in its...
-
The net income reported on the income statement for the current year was $132,000. Depreciation recorded on store equipment for the year amounted to $21,800. Balances of the current asset and current...
-
Use the results of Exercise 8.89 to show that where F depends on numerator df = [(Sample size for numerator sample variance) - 1] and denominator df = [(sample size for denominator sample variance) -...
-
1-3. What four factors are needed for marketing to occur?
-
Refer to Problem 8.3. If the final destination is New Delhi, India, and there is a 30% import tax, which firm should you choose? In Problem 8.3, you have been asked to analyze the bids for 200...
-
Need help, struggling to calculate, thank you!! Accounting for profit loss in the futures market, what is the effective price per bachel called the serie per bushel, you receive for your soybeans (8...
-
The production of steel from iron ore is endothermic. To reduce the heat that must be supplied, engineers need to find the lowest temperature at which the desired reactions are spontaneous. Estimate...
-
The standard entropy of vaporization of acetone is approximately 85 J K 1 mol 1 at its boiling point. (a) Estimate the standard enthalpy of vaporization of acetone at its boiling point of 56.2 C....
-
What is ODBC? How is it related to SQL/CLI?
-
Home Base, Incorporated reports the following production cost information: Units produced 97,000 units Units sold 92,000 units Ending finished goods inventory 5,000 units Direct labor $17 per unit...
-
About New York City public sector finance. The other way is to delineate the problem. We should use data to show a problem, and then analyze the environment in which budgeting takes place to suggest,...
-
From a survey a company has determined that 23% of its customers are classified as "advocates" , 68% as "passives" and the remainder as "detractors" . Research suggests that during a year 15% of the...
-
The following are the transactions of Spotlighter, Incorporated, for the month of January. a. Borrowed $3,940 from a local bank on a note due in six months. b. Received $4,630 cash from investors and...
-
1. What are the deeper problems that plague in different forms it takes throughout the world according to the authors? Please, briefly explain. 2. Why was Joseph Schumpeter a pessimist about the...
-
The Kf for the complex ion formation between Pb2+ and EDTA4- Ph" + EDTA" Pb(EDTA)2
-
In a large midwestern university, 30% of the students live in apartments. If 200 students are randomly selected, find the probability that the number of them living in apartments will be between 55...
-
Chromium compounds exhibit a variety of bright colors. When solid ammonium dichromate, (NH 4 ) 2 Cr 2 O 7 , a vivid orange compound, is ignited, a spectacular reaction occurs, as shown in the two...
-
Caffeine, a stimulant found in coffee, tea, chocolate, and some medications, contains 49.48% carbon, 5.15% hydrogen, 28.87% nitrogen, and 16.49% oxygen by mass and has a molar mass of 194.2....
-
A white powder is analyzed and found to contain 43.64% phosphorus and 56.36% oxygen by mass. The compound has a molar mass of 283.88g. What are the compounds empirical and molecular formulas?
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


