Calculate DH for the reaction Given the following data: 2NH3(g) + O(g) NH4(1) + HO(1)
Question:
Calculate DH for the reaction
Given the following data:![]()
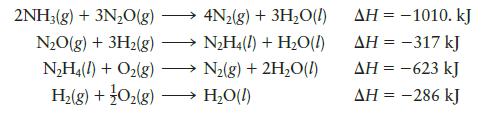
Transcribed Image Text:
2NH3(g) + O₂(g) N₂H4(1) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Answer DH 947 kJ...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From the data in Appendix 3, calculate DH for the synthesis of NO (which is the first step in the manufacture of nitric acid) at 25C: 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(l)
-
Calculate DH for the reaction given the following data: NH (1) + O(g) N(g) + 2HO(l)
-
For each data set, calculate s2. (a) (b) (c) -2 3 0 2 9 8 8 9 8 8 9
-
In the figure below, a square of edge lengths is formed by four spheres of masses, m, M, m3, and m4. What is the x component and the y component of the net gravitational force from them on a central...
-
Consider again the conditions of Exercise 7. Describe how to carry out a one-sided Wilcoxon-Mann-Whitney ranks test of the following hypotheses: H0: 0, H1: > 0.
-
When ammonia is first added to a solution of copper(II) nitrate, a pale blue precipitate of copper(II) hydroxide forms. As more ammonia is added, however, this precipitate dissolves. Describe what is...
-
What are the degrees of intensity of distribution? Provide an example for each type of intensity.
-
Consider an employee who does not receive employer-based health insurance and must divide her $700 per week in after-tax income between health insurance and other goods. Draw this workers opportunity...
-
Hudak Company requires a minimum cash balance of $ 4 , 6 0 0 . When the company expects a cash deficiency, it borrows the exact amount required on the first of the month. Expected excess cash is used...
-
You are a member of an independent consulting firm that specializes in serving the restaurant industry. Unlike many consulting firms that are extensions of audit firms, your firm has serious and in...
-
Given the following data: calculate DH for the reaction On the basis of enthalpy change, is this a useful reaction for the synthesis of ammonia? (g) + N (g) = 92 kJ (g) = 484 kJ
-
Consider the dissolution of CaCl 2 : CaCl(s) Ca+ (aq) + 2Cl(aq) AH = -81.5 kJ
-
A thermistor is a temperature-sensing element composed of a semiconductor material, which exhibits a large change in resistance proportional to a small change in temperature. A particular thermistor...
-
Watch the clip from HBO's Chernobyl and respond to the questions below using complete sentences....
-
1) Every organization has a unique culture. As you move forward with a travel or per diem position, what steps will you take to learn about your assignment organization's culture? 2)Flexibility and...
-
See the right figure of the lifting and transporting equipment. During operation, while a mass of 1.5 x 104 kg was descending at a constant speed v = 15 m/min, the machine experienced a breakdown,...
-
PROBLEM 3 BABEY Company makes three products with the following characteristics: Product V jk jin Selling price per unit 10 15 20 Variable cost per unit 6 10 10 Machine hours per unit 2 4 10 The...
-
Diversified Semiconductors sells perishable electronic components. Some must be shipped and stored in reusable protective containers. Customers pay a deposit for each container received. The deposit...
-
You are at a rock concert and find that when you stand 10 m from a speaker, the intensity is so high that it hurts your ears. You decide to move away from the stage so that your ears are more...
-
Cleaning Service Company's Trial Balance on December 31, 2020 is as follows: Account name Debit Credit Cash 700 Supplies Pre-paid insurance Pre-paid office rent Equipment Accumulated depreciation -...
-
For a hydrogen atom in its ground state, calculate the relative probability of finding the electron in the area described. a. In a sphere of volume 1.0 10-3 pm3 centered at the nucleus b. In a...
-
The treatment of a particle in a one-dimensional box can be extended to a rectangular box of dimensions Lx, Ly, and Lz, yielding the following expression for energy: The three quantum numbers nx, ny,...
-
Assume that eight electrons are placed into the allowed energy levels of a cubic box where two electrons can occupy each allowed energy level. (See Exercise 148 for the appropriate energy equation.)...
-
Production numbers for 2 shifts are shown. The shift supervisor of Shift 2 insists to the production manager that her operators are more productive than the ones on Shift 1. Using a confidence level...
-
In a class, the scores that students got are as shown. What are the 25, 50, 75 and 100th percentiles for the data? 84 84 98 80 89 83 85 56 85 84 84 74 84 81 83 80 45 86 67 79 81 78 76 85 83 77 86 83...
-
Number of points made by Teams A and B are shown. Which statement is true based on running the F-Test Two-Sample for Variances in the Data Analysis pack in Excel? Use a confidence level of 10% to...

Study smarter with the SolutionInn App


