Calculate S for the reduction of aluminum oxide by hydrogen gas using the following standard entropy values.
Question:
Calculate ΔS° for the reduction of aluminum oxide by hydrogen gas
![]()
using the following standard entropy values.
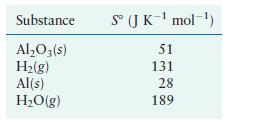
Transcribed Image Text:
AlO3(s) + 3H(g) 2Al(s) + 3HO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 20% (5 reviews)
AS Sproducts Sreactants 25 Als 3H...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Zirconium is one of the few metals that retains its structural integrity upon exposure to radiation. For this reason, the fuel rods in most nuclear reactors are made of zirconium. Answer the...
-
Many important biological reactions involve electron transfer. Because the pH of bodily fluids is close to 7, the biological standard potential of an electrode, E*, is measured at pH = 7....
-
Entropy can be calculated by a relationship proposed by Ludwig Boltzmann: S = kB ln V where kB = 1.38 Ã 1023 J/K and V is the number of ways a particular state can be obtained. (This equation...
-
Suppose a city finds that its express highways into the city are congested and it is considering two remedies: (1) imposing a congestion charge on all users of its expressways during the peak periods...
-
In the blizzard of 88, a rancher was forced to drop hay bales from an airplane to feed her cattle. The plane flew horizontally at 160 km/hr and dropped the bales from a height of 80m above the flat...
-
Describe the three segments of a supply chain?
-
What do the developers gain?
-
A small hotel in a popular resort area has 20 rooms. The hotel manager estimates that 15% of all confirmed reservations are no-shows. Consequently, the hotel accepts confirmed reservations for as...
-
1. Macs Caf uses a perpetual inventory system. The records reflected the following for January 2018: Units Unit Costs Beginning inventory 1,000 $55 Purchase, January 6 1,500 60 Purchase, January 14...
-
Consider the reaction carried out at 25C and 1 atm. Calculate H S, and G using the following data: 2SO2(g) + O2(g) 2SO3(g)
-
Predict the sign of S for each of the following reactions. a. the thermal decomposition of solid calcium carbonate: b. the oxidation of SO 2 in air: CaCO3(s) CaO(s) + CO,(g)
-
Describe the six steps in the selling process and what they entail.
-
In Exercises 29 and 30, find the probabilities and indicate when the "5% guideline for cumbersome calculations" is used. 29. Medical Helicopters In a study of helicopter usage and patient survival,...
-
Introduction to Internetworking Project 1: Ctrl-Alt-Del Inc. INTRODUCTION You have accepted a contract to participate in the design of the network infrastructure of a company called Ctrl-Alt-Del Inc....
-
Construct Arguments Tell whether each statement is always true, sometimes true, or never true. Explain. a. An integer is a whole number. b. A natural number is a rational number. c. An irrational...
-
Please answer the following Questions : 1. Who are the competitors for Whole Foods? 2. Do you consider traditional supermarkets to be competitors for natural and organic supermarkets? 3. How would...
-
LNC Corp is trying to determine the effect of its inventory turnover ratio and DSO on its cash conversion. Credit sales in 2016 is $101,000, cost of goods sold will be 70% of sales and it earned a...
-
Atmospheric air enters the air compressor of a simple combined gas-steam power system at 14.7 psia and 80F. The air compressor's compression ratio is 10; the gas cycle's maximum temperature is 2100F;...
-
Suppose that the electrical potential at the point (x, y, z) is E(x, y, z) = x + y - 2z. What is the direction of the acceleration at the point (1,3,2)?
-
The pre-equilibrium and the steady-state approximations are two different approaches to deriving a rate law from a proposed mechanism. For the following mechanism, determine the rate law (a) By the...
-
(a) What is the overall reaction for the following mechanism? (b) Write the rate law based on this mechanism. (c) How will the reaction rate depend on the pH of the solution? (d) How would the rate...
-
Write the formulas for the oxoanions of the following elements in which the element is found with its highest oxidation number (see Fig. 9A.7). In each case, the charge of the oxoanion is given in...
-
Difference between Operating Leverage and Financial Leverage
-
bpmn diagram for misc purchases
-
You have $55,000. You put 15% of your money in a stock with an expected return of 10%, $38,000 in a stock with an expected return of 18%, and the rest in a stock with an expected return of 22%. What...

Study smarter with the SolutionInn App


