Calculate the concentration of (mathrm{Pb}^{2+}) in each of the following. a. a saturated solution of (mathrm{Pb}(mathrm{OH})_{2} ;
Question:
Calculate the concentration of \(\mathrm{Pb}^{2+}\) in each of the following.
a. a saturated solution of \(\mathrm{Pb}(\mathrm{OH})_{2} ; K_{\mathrm{sp}}=1.2 \times 10^{-15}\)
b. a saturated solution of \(\mathrm{Pb}(\mathrm{OH})_{2}\) buffered at \(\mathrm{pH}=\) 13.00
c. \(0.010 \mathrm{~mol}\) of \(\mathrm{Pb}\left(\mathrm{NO}_{3}ight)_{2}\) added to \(1.0 \mathrm{~L}\) of aqueous solution, buffered at \(\mathrm{pH}=13.00\) and containing \(0.050 \mathrm{M} \mathrm{Na}_{4}\) EDTA. Does \(\mathrm{Pb}(\mathrm{OH})_{2}\) precipitate from this solution? For the reaction,
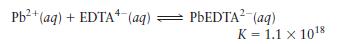
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





