Calculate the heat evolved or absorbed when 10.0 g of (a) NaCl; (b) NaI; (c) AlCl 3
Question:
Calculate the heat evolved or absorbed when 10.0 g of
(a) NaCl;
(b) NaI;
(c) AlCl3;
(d) NH4NO3 is dissolved in 100. g of water. Assume that the enthalpies of solution in Table 5D.3 are applicable and that the specific heat capacity of the solution is 4.18 J · K–1 · g–1.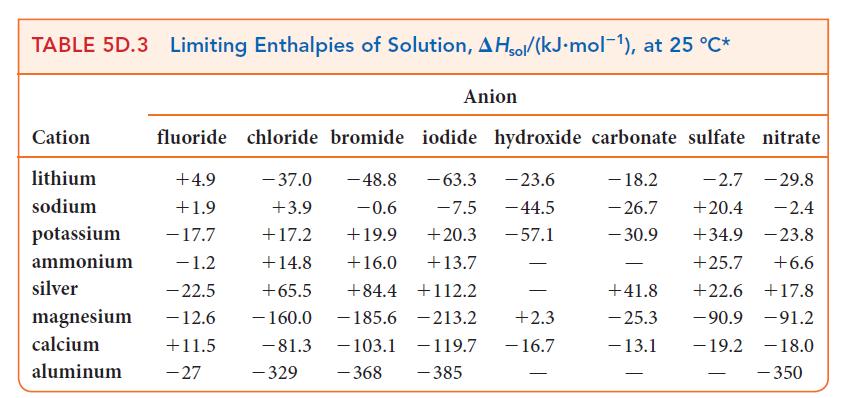
Transcribed Image Text:
TABLE 5D.3 Limiting Enthalpies of Solution, AHsol/(kJ.mol-¹), at 25 °C* Cation lithium sodium potassium ammonium silver magnesium calcium aluminum fluoride chloride bromide iodide hydroxide carbonate sulfate nitrate +4.9 - 37.0 -48.8 - 63.3 -23.6 - 18.2 -2.7 - 29.8 +1.9 +3.9 -0.6 -7.5 - 44.5 - 26.7 +20.4 -2.4 - 17.7 +17.2 +19.9 +20.3 -57.1 - 30.9 +34.9 -23.8 - 1.2 +14.8 +16.0 +13.7 +25.7 +6.6 -22.5 +65.5 +84.4 +112.2 +22.6 +17.8 - 12.6 - 160.0 -185.6 213.2 -90.9 -91.2 +11.5 -19.2 -103.1 - 119.7 -368 -385 -27 - 81.3 Anion -329 - +2.3 - 16.7 - +41.8 - 25.3 - 13.1 -18.0 - 350
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a 067 k...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In Problems 1922, use the method of Example 5 to find the constants A, B, and C in the indicated partial-fraction decompositions. 2 x(x - 1) X + B x-1 + C x + 1
-
In Exercises determine whether the statement is true or false. If it is false, explain why or give an example that shows it is false. lim x->0 x + ] x + x + 1 X = lim x-0 2x + 1 1 1
-
In Exercises 27 through 32, find the expected value for the random variable with the density function given in the indicated problem. a. P(1 X b. P(1 X 2)c. P(X 2) 3 f(x) = {x 0 if x 1 if x < 1
-
In Problems 1318, express the graph shown in blue using interval notation. Also express each as an inequality involving x. -1 0 1 2 3
-
A parallel combination of an 8- resistor and an unknown resistor R is connected in series with a 16- resistor and a battery. This circuit is then disassembled and the three resistors are then...
-
Regard an n x n matrix as a point in the -fold product Rn x . x Rn by considering each row as a member of Rn.. a. Prove that det : Rn x . x Rn Rn is differentiable and b. If aij : R R are...
-
Tim Allen Co. had sales revenue of $540,000 in 2004. Other items recorded during the year were: Prepare a single-step income statement for Allen for 2004. Allen has 100,000 shares of stock...
-
Refer to Problem 16-1. What additional funds would be needed if the companys year-end 2008 assets had been $4 million? Assume that all other numbers are the same. Why is this AFN different from the...
-
Understanding that the NPV capital budgeting technique is generally preferred by the authors of the text, why do other techniques continue to be used in practice? Give examples and reasons.
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
(a) Calculate the reaction Gibbs free energy of N 2 (g)+ 3 H 2 (g) 2 NH 3 (g) when the partial pressures of N 2 , H 2 , and NH 3 are 4.2 bar, 1.8 bar, and 21 bar, respectively, and the temperature...
-
Colligative properties can be sources of insight into not only the properties of solutions, but also the properties of the solute. For example, acetic acid, CH 3 COOH, behaves differently in two...
-
What is the speed parameter for the following speeds? (a) A typical rate of continental drift (1 in./y); (b) A typical drift speed for electrons in a current-carrying conductor (0.5 mm/s); (c) A...
-
The requirement for extended disclosures for oil and gas reserves described in Chapter 2 followed a Congressional hearing on the poor disclosures that Shell Oil had for its reserves. A.Explain three...
-
Question 9 Big Data techniques implemented in the financial sector include: fraud detection O marketing email campaign O customer relationship management techniques O inventory analysis
-
Problem 8-19A Attaining notfonpmt entity variances The Redmond Management Association held its annual public relations luncheon in April Year 2. Based on the previous year's results, the organization...
-
Kay, who is not a real estate dealer, sold an apartment house to Polly during the current year (2020). The closing statement for the sale is as follows. Total selling price $190,000 Add: Polly's...
-
1 English Writing Requirement Assignment Guidelines Sem 1 2023-24 Subject code AAE1D02 Subject title Introduction to Space Exploration Credit value 3 CAR Teachers Prof. WEN Chih-Yung, Prof. WU Bo,...
-
An open tank in a petroleum company lab contains a layer of oil on top of a layer of water. The water height is 4 times the oil height h. The oil has a specific gravity of 0.82. If the gauge pressure...
-
If there is an unrealized holding gain on available-for-sale investments, it is reported as?
-
Show that the expression (U/V) T = T(P/T)V P can be written in the form 2 / av
-
A sample containing 2.50 mol of an ideal gas at 325 K is expanded from an initial volume of 10.5 L to a final volume of 60.0 L. Calculate G and A for this process for a. An isothermal reversible path...
-
Why does the liquidgas coexistence curve in a PT phase diagram end at the critical point?
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


