Calculate the reaction quotient, Q, for the following cell reactions, given the measured values of the cell
Question:
Calculate the reaction quotient, Q, for the following cell reactions, given the measured values of the cell potential. Balance the chemical equations by using the smallest whole-number coefficients.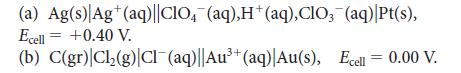
Transcribed Image Text:
(a) Ag(s) Ag+ (aq)||CIO4(aq),H+ (aq), CIO, (aq) |Pt(s), Ecell = +0.40 V. (b) C(gr) Cl₂(g) Cl¯(aq)||Au³+ (aq)| Au(s), Ecell = 0.00 V.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Ags Agaq ClO4aq Haq ClO3aq Pts Ecell 040 V Halfreactions Cathode Agaq e Ags E 0799 V Anode 6ClO3aq 6...View the full answer

Answered By

Ruksana K S
I have 2 year of experience in this field. I am engaged with reading, writing and my students as a child. Learning and teaching is part of my life.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the reaction quotient, Q, for the following cell reactions, given the measured values of the cell potential. Balance the chemical equations by using the smallest whole-number coefficients....
-
The oxidation of SO 2 to SO 3 is one of the reactions involved in the formation of acid rain. If you want to predict the spontaneous direction of the reaction for a specific mixture of the gases, you...
-
An electrochemical cell is set up using the following unbalanced reaction: Ma+(aq) + N(s) N2+(aq) + M(s) The standard reduction potentials are Ma+ + ae M o = 10.400 V N2+ + 2e- N o = 10.240 V The...
-
Mookie The Beagle Concierge Trial Balance As of January 31,2023 is given 1001 Checking 1010 Money Market 1100 Accounts Receivable (A/R) 1210 Prepaid Expenses:Supplies 1220 Prepaid Expenses:Insurance...
-
Partial productivity measurement Guble Company manufactures wallets from fabric. In 2008, Guble made 2,500,000 wallets using 1,875,000 yards of fabric. In 2009, Guble plans to make 2,650,000 wallets...
-
A red train traveling at 72km/h and a green train traveling at 144km/h arc headed toward each other along a straight, level track. When they are 950 m apart, each engineer sees the other's train and...
-
Think of both a successful project and an unsuccessful project with which you are familiar. What distinguishes the two, both in terms of the process used to develop them and their outcomes?
-
Chloe and Emma start a new business, Cement Sidewalks and Accessories (CSA), during the current year. CSA is organized as a partnership. Chloe owns 40% of CSA; Emma owns the remaining 60%. Chloe and...
-
A bond pays annual interest. Its coupon rate is 9.8%. Its value at maturity is $1,000. It matures in 4 years. Its yield to maturity is currently 6.8%. The duration of this bond is _______ years.
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The pH of 0.50 m HBrO(aq) is 4.50. Calculate the change in pH when 5.10 g of sodium hypobromite is added to 100. mL of the solution. Ignore any change in volume.
-
A large volume of 0.250 m H 2 S(aq) is treated with a strong base to adjust the pH to 9.35. Assume that the addition of the base, a solid, does not significantly affect the volume of the solution....
-
The algorithms for insertion and deletion into a B+ tree are presented as recursive algorithms. In the code for insert, for instance, a call is made at the parent of a node N to insert into (the...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Identifying Binomial Distributions. Determine whether the given procedure results in a binomial distribution or a distribution that can be treated as binomial (by applying the 5% guideline for...
-
Case 6: TOMS Shoes in 2016: An Ongoing Dedication to Social Responsibility, by Margaret A. Peteraf, Sean Zhand, and Meghan L. Cooney (page C-57) Read the case and then respond to the case questions...
-
Quatro Co. issues bonds dated January 1, 2019, with a par value of $740,000. The bonds' annual contract rate is 13%, and interest is paid semiannually on June 30 and December 31. The bonds mature in...
-
Wildcat Mining wants to know the appropriate discount rate to use in their capital budgeting decision making process. Based on the following data, what is the weighted average cost of capital the CFO...
-
In a plant mitigation project, an entire local (endangered) population of 255 Congdon's tar plants was transplanted to a new location.35 One year after transplant, 30 of the 255 plants were randomly...
-
As water moves through the hydrologic cycle, water quality changes are common because of natural phenomena or anthropogenic pollution. Using Figure 11.1, describe how water-quality changes occur...
-
If the total pressure is increased at constant T, how will the relative amounts of H 2 (g) and HCl(g) change? H 2 (g) + Cl 2 (g) 2HCl(g) at equilibrium. Assume ideal gas behavior.
-
Propose an efficient synthesis for each of the following transformations: a. b. c. d. Br
-
When methyl benzoate bears a substituent at the para position, the rate of hydrolysis of the ester moiety depends on the nature of the substituent at the para position. Apparently, a methoxy...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...

Study smarter with the SolutionInn App


