Calculate the standard entropy of vaporization of ammonia at 210.0 K, given that the molar heat capacities
Question:
Calculate the standard entropy of vaporization of ammonia at 210.0 K, given that the molar heat capacities at constant pressure of liquid ammonia and ammonia vapor are 80.8 J · K–1 · mol–1 and 35.1 J · K–1 · mol–1, respectively, in this range (see Table 4C.1).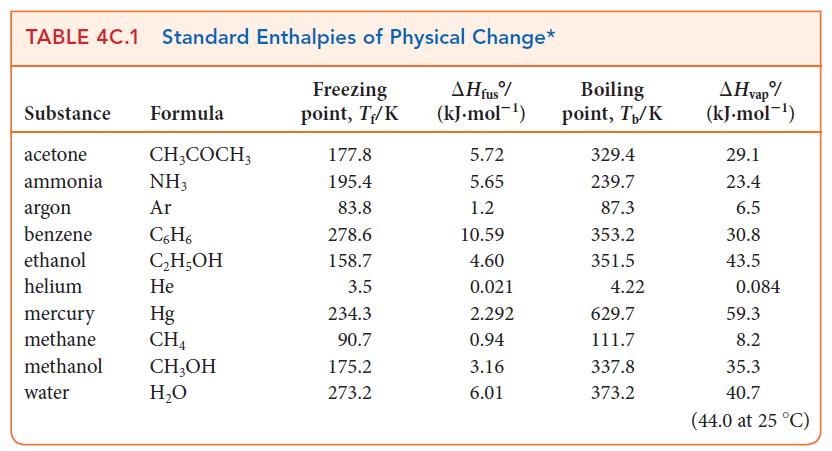
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





