Complete and balance each of the following equations: (a) FeS (s) + HCl(aq) (b) H(g) +
Question:
Complete and balance each of the following equations: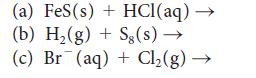
Transcribed Image Text:
(a) FeS (s) + HCl(aq) → (b) H₂(g) + Sg(s) → (c) Br(aq) + Cl₂(g) →
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Completed and balanc...View the full answer

Answered By

SHIVAM SHUKLA
I TEACH STUDENTS IN MY HOME FROM THE LAST THREE YEARS
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Complete and balance each of the following molecular equations (in aqueous solution); include phase labels. Then, for each, write the net ionic equation. a. NaOH + HNO3 b. HCl + Ba(OH)2 c. HC2H3O2...
-
Complete and balance each of the following molecular equations (in aqueous solution); include phase labels. Then, for each, write the net ionic equation. a. Al(OH)3 + HCl b. HBr + Sr(OH)2 c....
-
Complete and balance each of the following molecular equations, including phase labels, if a reaction occurs. Then write the net ionic equation. If no reaction occurs, write NR after the arrow. a....
-
Which sensing structure helps a company test and make sense of new ideas generated through other types of sensing? Social listening Crowdsourcing Blue ocean Pilot test
-
Does the following manifesto make sense? Explain briefly. Were a darn successful company. Our book rate of return has exceeded 20 percent for five years running. Were determined that new capital...
-
Determine the convolution of the following pairs of signals by means of the z- transform. (a) x1(n) = (1/4)nu(n - 1), x2(n) = [1 + (1/2)n]u(n) (b) x1(n) = u(n), x2 (n) = (n) + (1/2)n u(n) (c) x1(n) =...
-
1. Income and Substitution Effects. Sabrina works for a workers cooperative that initially pays her a lump sum of $200 per week (as long as she works at least 15 hours per week) and a wage of $20 per...
-
Suppose the coupon rate for a TIPS is 2.6% and that youve just today purchased a new $10,000 face value issue. Assume that the annual inflation rate over the next year is 2.2%. a) After 6 months,...
-
Complete and balance the following equations: (a) AlO3(s) + OH (aq) (b) AlO3(s) + H3O+(aq) + HO(1) (c) B(s) + NH3(g)
-
(a) Nitrous acid reacts with hydrazine in acidic solution to form hydrazoic acid, HN 3 . Write the chemical equation and determine the mass of hydrazoic acid that can be produced from 15.0 g of...
-
Independent random samples are selected from two populations. The data are shown in the table. a. Use the Wilcoxon rank sum test to determine whether the data provide sufficient evidence to indicate...
-
(25 Points) University Painting is considering investing in a new paint sprayer to allow them to paint more classrooms in less time. The sprayer would have the following cash flow and cost of capital...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Summit Regional Medical Center operates as a private not-for-profit hospital, providing services to a community of 20,000 and the surrounding rural areas. Summit has maintained a banking relationship...
-
Woodland Wearables produces two models of a smart watch, the Basic and the Flash. The watches have the following characteristics: Basic Flash Selling price per watch $ 3 3 0 $ 4 9 0 Variable cost per...
-
Introduction This practice case has been designed to give introductory-level business students practical experience in the application of accounting concepts. This practice case will provide students...
-
Consider a simple model to estimate the effect of personal computer (PC) ownership on college grade point average for graduating seniors at a large public university: GPA = 0 + 1PC + u, where PC is a...
-
Cassandra Casey operates the Futuristic Antique Store. She maintains subsidiary ledgers for accounts payable and accounts receivable. She presents you with the following information for October 2019:...
-
The molecular electrostatic potential maps for LiH and HF are shown here. Does the apparent size of the hydrogen atom (shown as a white sphere) tell you whether it is an electron acceptor or an...
-
For H + 2 , explain why H aa is the total energy of an undisturbed hydrogen atom separated from a bare proton by the distance R.
-
Distinguish between the following concepts used to describe chemical bond formation: basis set, minimal basis set, atomic orbital, molecular orbital, and molecular wave function.
-
THIS IS ONE QUESTION WITH TWO PARTS. PLEASE ANSWER COMPLETELY AND SHOW ALL WORK. (NO EXCEL) Information for Question 1: State Probability Retum on A Return on B Return on C Retum on Portfolio X Boom...
-
Direct materials (5.0 Ibs. @ $5.00 per Ib.) Direct labor (2.0 hrs. @ $13.00 per hr.) Overhead (2.0 hrs. @ $18.50 per hr.) Total standard cost $25.00 26.00 37.00 $88.00 The predetermined overhead rate...
-
Problem 1-28 (Algo) (LO 1-4, 1-5, 1-6b 1-7) Harper, Inc., acquires 40 percent of the outstanding voting stock of Kinman Company on January 1, 2020, for $316,100 in cash. The book value of Kinman's...

Study smarter with the SolutionInn App


